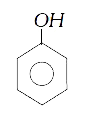
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The ionization constant of a phenol is higher than that of ethanol bec...
Text Solution
|
- Ethanol has higher boiling point than ethanol because
Text Solution
|
- The ionization constant of a phenol is higher than that of ethanol bec...
Text Solution
|
- The ionisation constant of phenol is higher than that of ethanol becau...
Text Solution
|
- फीनॉल का आयनन स्थिरांक एथेनॉल से अधिक होता है क्योंकि:
Text Solution
|
- नाइट्रोजन की आयनन ऊर्जा ऑक्सीजन की आयनन ऊर्जा से अधिक, क्योकिं
Text Solution
|
- The ionization constant of phenol is higher than that of ethanol becau...
Text Solution
|
- The ionisation constant of phenol is higher than that of ethanol becau...
Text Solution
|
- नाइट्रोजन की प्रथम आयनन ऊर्जा का मान ऑक्सीजन से अधिक होता है, इसका कार...
Text Solution
|