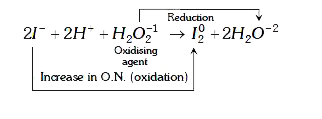A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN
ERRORLESS|Exercise PAST YEARS QUESTIONS |16 VideosHYDROGEN
ERRORLESS|Exercise ASSERTION & REASON|6 VideosHYDROGEN
ERRORLESS|Exercise NCERT BASED QUESTION (Water or Hydride of Oxygen)|42 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 VideosIONIC EQUILIBRIUM
ERRORLESS|Exercise ASSERTION AND REASON |9 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-HYDROGEN-NCERT BASED QUESTION (Hydrogen Peroxide)
- Consider the following reactions which are carried out at the same tem...
Text Solution
|
- The oxide that gives H2 O2 on treatment with dilute H2 SO4 is
Text Solution
|
- Which of the following equations depicts the oxidizing nature of H2O2
Text Solution
|
- Which of the following equation depicts reducing nature of H(2)O(2)...
Text Solution
|
- When sodium peroxide is trated with the dilute sulphuric acid, we...
Text Solution
|
- Hydrogen peroxide is obtained by the electrolysis of ………
Text Solution
|
- There is a smaple of 10 volume of hydrogen peroxide solution . Calcula...
Text Solution
|
- The structure of H2O2 is
Text Solution
|
- H2O2 acts as an oxidising agent in
Text Solution
|
- Blackened oil painting can be restored into original form by the actio...
Text Solution
|
- Hydrogen peroxide is reduced by
Text Solution
|
- What is the product of the reaction of H2O2 with Cl2
Text Solution
|
- Fenton’s reagent is
Text Solution
|
- In transforming 0.01 mole of PbS to PbSO(4), the volume of '10 volume ...
Text Solution
|
- The volume strength of 1.5 N H2 O2 solution is
Text Solution
|
- The strength in volumes of a solution containing 30.36 g/L of H(2)O(2)...
Text Solution
|
- H(2)O(2) is manufactured these days
Text Solution
|
- The O-O-H bond angle in H2 O2 is ?
Text Solution
|
- The laboratory method for the preparation of H(2)O(2) is by
Text Solution
|
- H2O2 is a
Text Solution
|