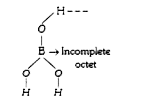A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise NCERT Based Questions (Carbon Family)|60 VideosTHE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise Past Yeat Questions|32 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 VideosTHE S-BLOCK ELEMENTS
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)-Assertion & Reason
- Boric acid is an acid because its molecule
Text Solution
|
- Assertion: Al(OH)(3) is insoluble in NH(4)OH but soluble in NaOH. Re...
Text Solution
|
- Assertion: Boron is metalloid. Reason : Boron shows metallic nature...
Text Solution
|
- These questions consist of two statements each, printed as Assertion a...
Text Solution
|
- Assertion : All forms [Alf(6)]^(3-) but B does not form [BF(6)]^(3-). ...
Text Solution
|
- Assertion: Benzene is reactive while inorganic benzene is unreactive ...
Text Solution
|
- Assertion: The first ionization energy of Be is greater than that of B...
Text Solution
|
- Assertion: SiF(6)^(2-) is known but SiCl(6)^(2-) is not. Reason: Siz...
Text Solution
|
- Assertion: Si-Si bonds are much weaker than Si-O bonds. Reason: Sili...
Text Solution
|
- Assertion (A): PbCl(2) is more stable than PbCl(4). Reason (R ): P...
Text Solution
|
- Assertion: Silicones are hydrophobic in nature. Reason: Si-O-Si link...
Text Solution
|