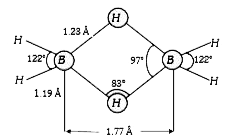
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise Assertion & Reason|10 VideosTHE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise NCERT Based Questions (Carbon Family)|60 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 VideosTHE S-BLOCK ELEMENTS
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)-Past Yeat Questions
- Which of the following statements about H(3)BO(3) is not correct ?
Text Solution
|
- Which of the following is the electron-deficient molecule?
Text Solution
|
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- Which of the following structure is similar to graphite e?
Text Solution
|
- Which of the following statements is incorrect
Text Solution
|
- Which one of following elements is unable to from MF(6)^(3-) ion?
Text Solution
|
- The ionic carbide is
Text Solution
|
- Which specie does not exist?
Text Solution
|
- Which of the following gives propyne on hydrolysis?
Text Solution
|
- Hydrolysis of which of the following does not occur?
Text Solution
|
- Supercritical CO(2) is used as
Text Solution
|
- Which of the following is not isostructural with SiCI(4) ?
Text Solution
|
- The basic structural unit of silicates is
Text Solution
|
- Which of the following glass is used in making wind screen of automobi...
Text Solution
|
- The veriety of glass used in making lenses and prisms is
Text Solution
|
- Which of the following statements about the zeolites is false?
Text Solution
|
- Which statement is wrong
Text Solution
|
- Non-oxide ceramics can be
Text Solution
|
- Name the type of the structure of silicate in which one oxygen atom of...
Text Solution
|
- Which of these is not a monomer for a high-molecular mass silicone pol...
Text Solution
|