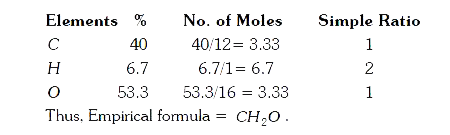A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PURIFICATION, CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTIONS (CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS) |100 VideosPURIFICATION, CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
ERRORLESS|Exercise PAST YEARS QUESTIONS|28 VideosIONIC EQUILIBRIUM
ERRORLESS|Exercise ASSERTION AND REASON |9 VideosREDOX REACTIONS
ERRORLESS|Exercise ASSERTION & REASON|6 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-PURIFICATION, CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-ASSERTION & REASON
- An organic compound with C =40 % and H= 6.7% will have the empirical f...
Text Solution
|
- Assertion : A mixture of plant pigments can be separated by chromatogr...
Text Solution
|
- Assertion : Moving phase is liquid and stationary phase is solid in pa...
Text Solution
|
- Assertion : Thiophene present in commercial benzene as an impurity can...
Text Solution
|
- Assertion : Refining of petroleum involves fractional distillation. ...
Text Solution
|
- Assertion : Potassium can be used in Lassaigne test. Reason : Potass...
Text Solution
|
- Assertion : is 3-methyl cyclopentene. Reason : In numbering, double...
Text Solution
|
- Assertion : During test for nitrogen with Lassaigne extract on adding ...
Text Solution
|
- Assertion: Magnetic resonance imaging (MRI) is a useful diagnostic too...
Text Solution
|
- (A) Oils are purified by steam distillation. (R) The compounds which...
Text Solution
|