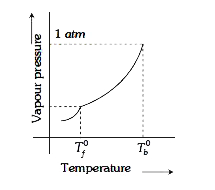
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (IDEAL AND NON-IDEAL SOLUTION)|16 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (AZEOTROPIC MIXTURE)|9 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (COLLIGATIVE PROPERTIES)|8 VideosSOLID STATE
ERRORLESS|Exercise ASSERTION & REASON|11 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLUTIONS -NCERT BASED QUESTIONS (LOWERING OF VAPOUR PRESSURE)
- A solution is obtained by dissolving 12gof urea(mol.wt.60)in a litre o...
Text Solution
|
- Equal weight of CH(4) " and " H(2) are mixed in an empty container at ...
Text Solution
|
- The boiling point of a solution of 0.11g of a substance is 15g of ethe...
Text Solution
|
- Two beakers of capacity 500 mL were taken. One of these beakers, label...
Text Solution
|
- An aqueous solution of methanol in water has vapour pressure
Text Solution
|
- Which solution will show the maximum vapour pressure at 300 K?
Text Solution
|
- The relative lowering of vapour pressure is equal to the ratio between...
Text Solution
|
- 5 cm^(3) of acetone is added to 100 cm^(3) of water . Then the va...
Text Solution
|
- A solution has 1:4 mole ratio of pentane to hexane . The vapour pressu...
Text Solution
|
- The vapour pressure lowering caused by addition of 100 g of sucrose (m...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- An ideal solution was obtained by mixing methanol and ethanol. If the ...
Text Solution
|
- Which of the following has maximum vapour pressure?
Text Solution
|
- If two substances A and B have p(A)^(@): p(B)^(@) = 1:2 and have mole ...
Text Solution
|
- The mass of glucose that would be dissolved in 50g of water in order t...
Text Solution
|
- Assertion (A): Vapour pressure increase with increase in temperature. ...
Text Solution
|
- Pick out the wrong statement (s). (i) Vapour pressure of a liquid ...
Text Solution
|
- Low concentration of oxygen in the blood ndtissues of people living at...
Text Solution
|
- When a liquid that is immiscible with water was steam distilled at 952...
Text Solution
|
- A liquid has a vapour pressure of 40mm Hg at 19.0^(@)C and a normal bo...
Text Solution
|