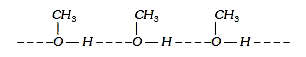A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (AZEOTROPIC MIXTURE)|9 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (OSMOSIS AND OSMOTIC PRESSURE OF THE SOLUTION)|36 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (LOWERING OF VAPOUR PRESSURE)|36 VideosSOLID STATE
ERRORLESS|Exercise ASSERTION & REASON|11 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLUTIONS -NCERT BASED QUESTIONS (IDEAL AND NON-IDEAL SOLUTION)
- A solution that shows negative deviation from Raoult’s Law among the f...
Text Solution
|
- v34
Text Solution
|
- One component of a solution follows Raoult's over the entire range 0le...
Text Solution
|
- Considering the formation, breaking and strength of hydrogen bond, pre...
Text Solution
|
- On the basic of information given below mark the Correct option .Infor...
Text Solution
|
- Which of the following liquid pairs shows a positive deviation from Ra...
Text Solution
|
- Identify the mixture that shows positive deviations from Raoult's law
Text Solution
|
- Which of the following solutions shows positive deviation from Raoult'...
Text Solution
|
- Which of the following will show a negative deviation from Raoult’s la...
Text Solution
|
- All form ideal solution except
Text Solution
|
- Which property is shown by an ideal solution?
Text Solution
|
- Which one of the following is non-ideal solution?
Text Solution
|
- When non-ideal solution was prepared by mixing 30 mL chloroform and 50...
Text Solution
|
- When two liquids A and B are mixed, their boiling points become greate...
Text Solution
|
- Under what condition do non-ideal solutions show negative deviations?
Text Solution
|
- Assuming ideal behaviour, the enthalpy and volume of mixing of two liq...
Text Solution
|