A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (ELEVATION OF BOILING POINT OF THE SOLVENT)|14 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (DEPRESSION OF FREEZING POINT OF THE SOLVENT) |22 VideosSOLUTIONS
ERRORLESS|Exercise NCERT BASED QUESTIONS (AZEOTROPIC MIXTURE)|9 VideosSOLID STATE
ERRORLESS|Exercise ASSERTION & REASON|11 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLUTIONS -NCERT BASED QUESTIONS (OSMOSIS AND OSMOTIC PRESSURE OF THE SOLUTION)
- What is the osmotic pressure of a 0.0020 mol dm^(-3) sucrose (C(12)H(2...
Text Solution
|
- For getting accurate value of molar mass of a solute by osmotic pressu...
Text Solution
|
- Desalination of sea water can be done by
Text Solution
|
- The osmotic pressure of a solution can be increased by
Text Solution
|
- The empirical formula of a non-electrolyte is CH(2)O. A solution conta...
Text Solution
|
- An unriped mango placed in a concentrated salt solution to prepare pic...
Text Solution
|
- Which of the following statements is false
Text Solution
|
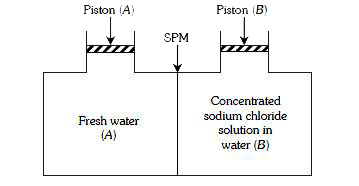
- Consider the figure and mark the correct option
Text Solution
|
- Osmotic pressure of 40% (wt.//vol.) urea solution is 1.64 atm and that...
Text Solution
|
- Blood is isotonic with:
Text Solution
|
- Which inorganic precipitate acts as semipermeable membrane ?
Text Solution
|
- The osmotic pressure of a solution can be increased by
Text Solution
|
- Which of the following associated with isotonic solutions is not corre...
Text Solution
|
- At low concentrations, the statement that equimoala solutions under a ...
Text Solution
|
- Solution A contains 7 g/L of MgCl(2) and solution B contains 7 g/L of...
Text Solution
|
- If molecular weight of compound is increased then sensitivity is decre...
Text Solution
|
- which of the following compounds is used as a semipermeable membrane?
Text Solution
|
- At a certain temperature, the value of the slope of the plot of osmoti...
Text Solution
|
- What will happen if an animal cell is placed in hypertonic solution ?
Text Solution
|
- At 298 K, the ratio of osmotic pressure of two solutions of a substanc...
Text Solution
|