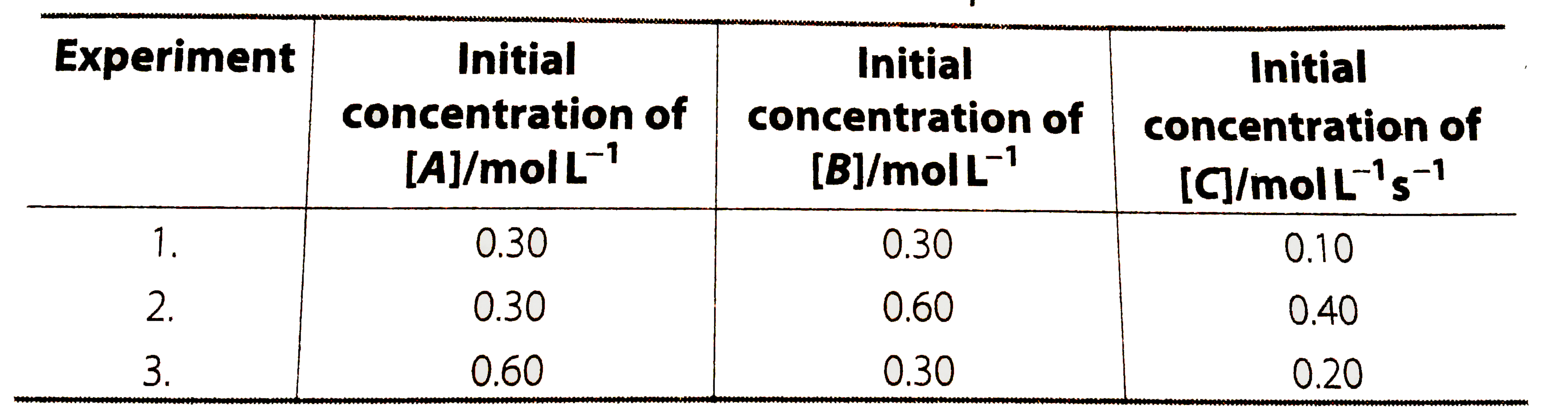
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Rate Law and Rate Constant)|106 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Collision Theory, Energy of Activation and Arrhenius Equation)|54 VideosCARBOXYLIC ACIDS
ERRORLESS|Exercise Assertion & Reason|5 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-Assertion & Reason
- Compounds 'A' and 'B' react according to the following chemical eq...
Text Solution
|
- Assertion: Glow worm shows chemiluminescence. Reason: Glow worm emit...
Text Solution
|
- Assertion : The rate of reaction is always negative . Reason : Minus...
Text Solution
|
- Assertion (A) : The order of a reaction can have fractional value Re...
Text Solution
|
- Assertion. Average life of a radioactive element is that period in whi...
Text Solution
|
- Assertion : The hydrolysis of methyl acetate by dil. HCl is a pseudo f...
Text Solution
|
- Assertion: The emission of light during burning of P in O(2) is called...
Text Solution
|