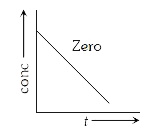
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Collision Theory, Energy of Activation and Arrhenius Equation)|54 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Photochemical Reaction)|4 VideosCHEMICAL KINETICS
ERRORLESS|Exercise Assertion & Reason|6 VideosCARBOXYLIC ACIDS
ERRORLESS|Exercise Assertion & Reason|5 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-NCERT BASED QUESTIONS (Rate Law and Rate Constant)
- The following data are for the decomposition of ammonium nitrite in aq...
Text Solution
|
- The thermal decomposition of a compound is of first order. If 50% of a...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- 75% of a first-order reaction was completed in 32 minutes, when was 50...
Text Solution
|
- Which of the following is not correct
Text Solution
|
- Cyclopropane rearranges to form propane : DeltararrCH(3)-CH=CH(2) ...
Text Solution
|
- Consider the decomposition of N(2)O(5) as N(2)O(5)rarr2NO(2)+1//2O(2...
Text Solution
|
- Sucrose decomposes in acid solution into glucose and fructose accordin...
Text Solution
|
- For the second order reaction, A+Brarr"products" when a moles of A...
Text Solution
|
- Consider a first order gas phase decomposition reaction given below: ...
Text Solution
|
- The following data is obtained during the first order thermal decompos...
Text Solution
|
- For consecutive first order reaction Aoverset(k(1))(to)Boverset(k(2)...
Text Solution
|
- The rate of the reaction A rarr products, at the initial concentration...
Text Solution
|
- X overset("Step"1)to Y overset("StepII")to Z is a complex reaction. ...
Text Solution
|
- Reaction : - 3ClO^(-) to ClO3^(-) +2Cl^(-) occurs in following t...
Text Solution
|
- The reaction,2NO(g)+O(2)(g)hArr2NO(2)(g), is of first order. If the vo...
Text Solution
|
- In many reaction the reaction proceeds in a sequence of steps so the o...
Text Solution
|
- For a chemical reaction A to B it is found that the rate of reaction ...
Text Solution
|
- The inversion of cane sugar is represented by C(12)H(22)O(11) + H(2)...
Text Solution
|
- The reaction 2N(2)O(5) hArr 2NO(2)+O(2) follows first order kinetics. ...
Text Solution
|