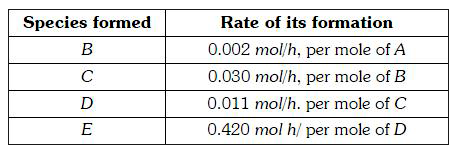
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Collision Theory, Energy of Activation and Arrhenius Equation)|54 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Photochemical Reaction)|4 VideosCHEMICAL KINETICS
ERRORLESS|Exercise Assertion & Reason|6 VideosCARBOXYLIC ACIDS
ERRORLESS|Exercise Assertion & Reason|5 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-NCERT BASED QUESTIONS (Rate Law and Rate Constant)
- If order of reaction A+B overset(hv)rarrAB is zero. It means that
Text Solution
|
- The rate of reaction A + 2B rarr 3C gets increased by 72 times when th...
Text Solution
|
- Given the hypothetical reaction mechanism Aoverset(I)(to)B overset(I...
Text Solution
|
- Rate of the given reaction, (1) A + B overset(r(1)=0.05) to X (2) ...
Text Solution
|
- The hydrolysis of ethyl acetate CH(3)COOC(2)H(5) + H(2)O overset(H^(+)...
Text Solution
|
- For the reaction O(3(g)) +O((g)) to 2O(2(g)) , if the rate law...
Text Solution
|
- For the reaction 2SO(2)+O(2)("excess") to 2SO(3) the order of reaction...
Text Solution
|
- Which of the following statement is not correct about order of a ...
Text Solution
|
- The value of rate constant of a pseudo first order reaction
Text Solution
|
- The reaction 2H2O2 to 2H2O + O2 is a
Text Solution
|
- For the reaction: H(2) + Cl(2) underset("Sunlight")rarr2HCl taking...
Text Solution
|
- The given reaction 2NO +O(2) rarr 2NO(2) is an example of
Text Solution
|
- Which of the following is an example of pseudo unimolecular reaction ?
Text Solution
|
- Which one the following statement for order of reactions is not correc...
Text Solution
|
- Which of the following reaction ends in finite time ?
Text Solution
|
- For a chemical reaction Ararr B,the rate of the reaction is 2.0xx10^(-...
Text Solution
|
- For a reaction between A and B, the initial rate of reaction is measur...
Text Solution
|
- Which of the following is the correct statement
Text Solution
|
- For the reaction, 2A + B to C + D, the order of reaction is
Text Solution
|
- Certain reactions follow the relation between concentrations of the re...
Text Solution
|