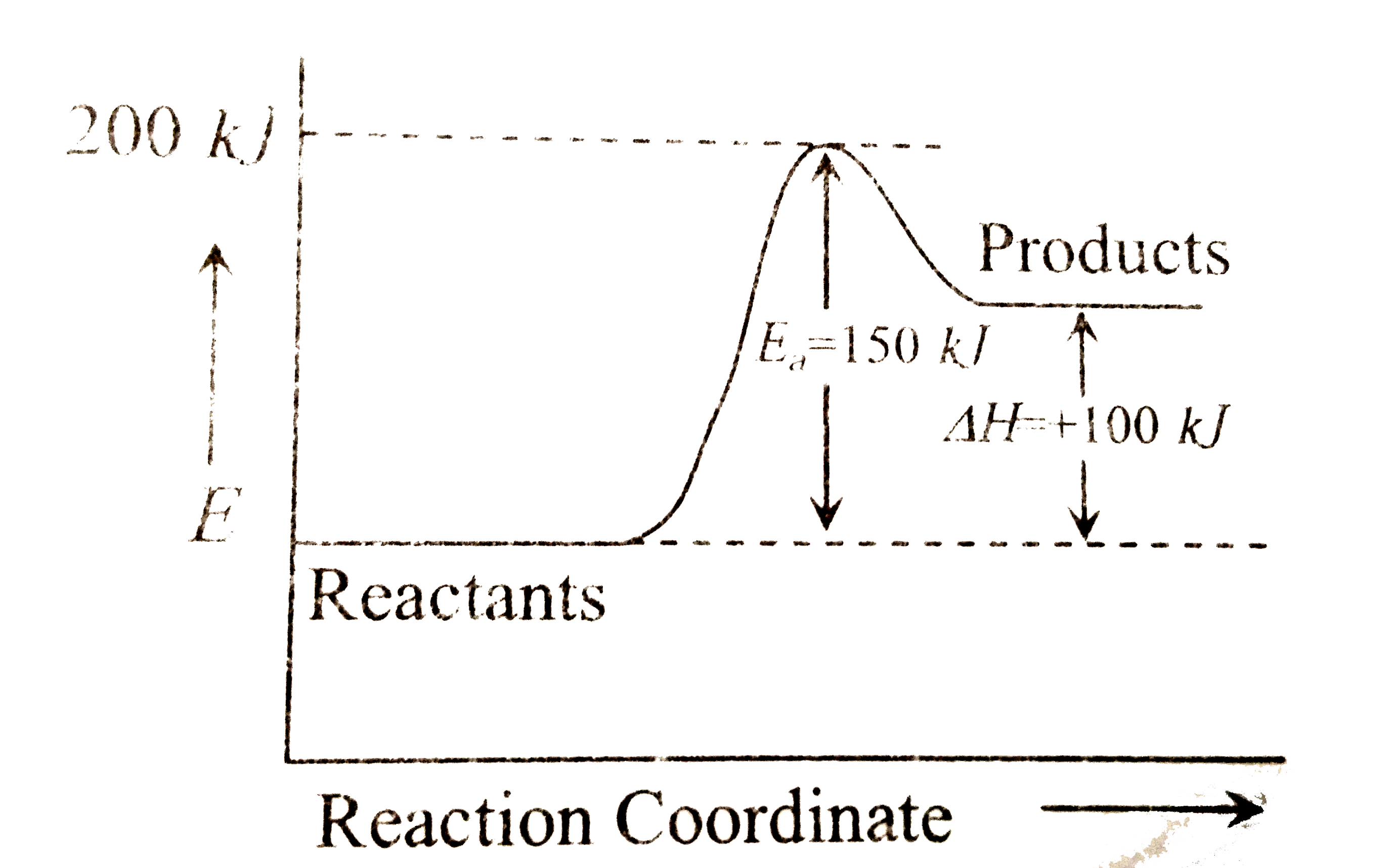
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Photochemical Reaction)|4 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Graphical Questions)|7 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Rate Law and Rate Constant)|106 VideosCARBOXYLIC ACIDS
ERRORLESS|Exercise Assertion & Reason|5 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-NCERT BASED QUESTIONS (Collision Theory, Energy of Activation and Arrhenius Equation)
- The rate constant is given by the equation k = P.Ze^(-E//RT). Which fa...
Text Solution
|
- Chemical reactions with very high E(a) values are generally
Text Solution
|
- In the given graph the activation energy , E(a) for the reverse reacti...
Text Solution
|
- If the activation enery for the forward reaction is 150 "kJ mol"^(-1) ...
Text Solution
|
- The rate constant (K') of one reaction is double of the rate constant ...
Text Solution
|
- The role of a catalyst is to change
Text Solution
|
- In the presence of a catalyst, the heat evolved or absorbed during the...
Text Solution
|
- For a reversible reaction where the forward reaction is exotherminc, w...
Text Solution
|
- A large increase in the rate of a reaction for a rise in temperature i...
Text Solution
|
- On increasing the temperature, the rate of the reaction increases beca...
Text Solution
|
- The minimum energy a molecule should possess in order to enter into a ...
Text Solution
|
- Activation energy is
Text Solution
|
- The reason for almost doubling the rate of reaction on increasing the ...
Text Solution
|
- The activation energy for a reaction at the temperature T K was found ...
Text Solution
|
- Collision theory is applicable to
Text Solution
|
- Pick the appropriate choice about collision theory of reaction rates
Text Solution
|
- The rate of reactions exhibiting negative activation energy
Text Solution
|
- Why do most chemical reaction rates increase rapidly as the temperatur...
Text Solution
|
- Which of the following statement is not correc for following straight ...
Text Solution
|
- The minimum energy required for molecules to enter into the reaction i...
Text Solution
|