A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Photochemical Reaction)|4 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Graphical Questions)|7 VideosCHEMICAL KINETICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Rate Law and Rate Constant)|106 VideosCARBOXYLIC ACIDS
ERRORLESS|Exercise Assertion & Reason|5 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-NCERT BASED QUESTIONS (Collision Theory, Energy of Activation and Arrhenius Equation)
- Which of the following statement is not correc for following straight ...
Text Solution
|
- The minimum energy required for molecules to enter into the reaction i...
Text Solution
|
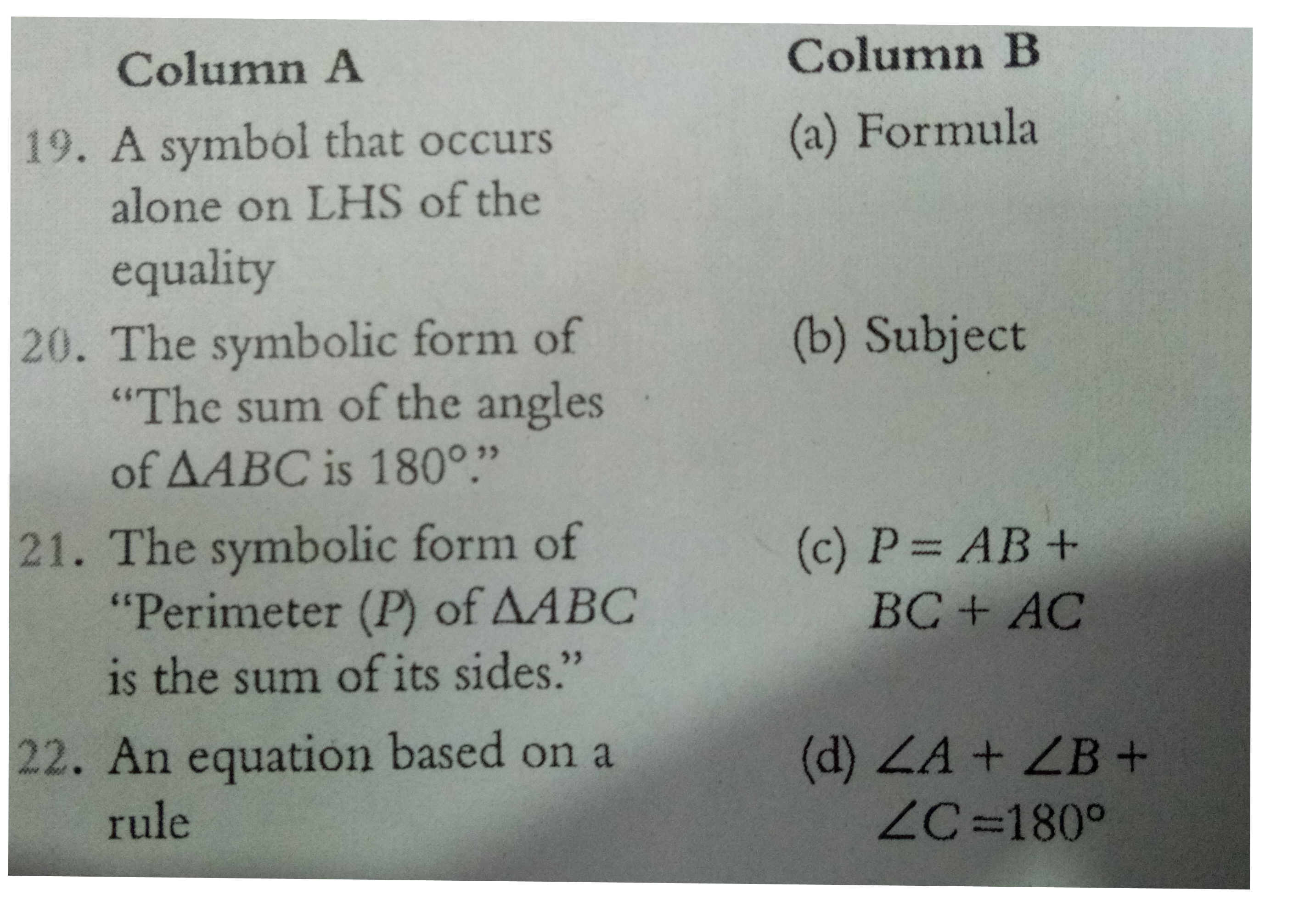
- Match the following Column A to Column B
Text Solution
|
- Which of the following are correct chain isomers of butane ? (i) <im...
Text Solution
|
- Which of the following statements is incorrect about the collision the...
Text Solution
|
- Which of the following statement is not correct for the catalyst ...
Text Solution
|
- A chemical reaction was carried out at 300 K and 280 K. The rate const...
Text Solution
|
- Transition state 2 (T.S.2) is structurally most likely as
Text Solution
|
- Which graph shows zero activation energy ?
Text Solution
|
- If a homogeneous catalytic reaction can take place through three alter...
Text Solution
|
- In Arrhenius plot, intercept is equal to
Text Solution
|
- ARRHENIUS THEORY
Text Solution
|
- The Arrhenius equation expressing the effect of temperature on the rat...
Text Solution
|
- The rate constant of a reaction at temperature 200 K is 10 times less ...
Text Solution
|
- In respect of the equation k=Ae^(-E(a)//RT) in chemical kinetics, whic...
Text Solution
|
- Activation energy (E(a)) and rate constants (k(1) and k(2)) of a chemi...
Text Solution
|
- The specific rate constant for a reaction increases by a factor of 4, ...
Text Solution
|
- The rate temperature changes from 300K to 310K. Activation energy of s...
Text Solution
|
- The activation energy of a reaction can be determined from the slope o...
Text Solution
|
- The temperature dependence of rate constant (k) of a chemical reaction...
Text Solution
|