A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-PAST YEARS QUESTIONS
- If doubling the concentration of a reactant 'A' increases the rate 4 t...
Text Solution
|
- The rate of disappearance of SO(2) in the reaction 2SO(2) + O(2) rarr ...
Text Solution
|
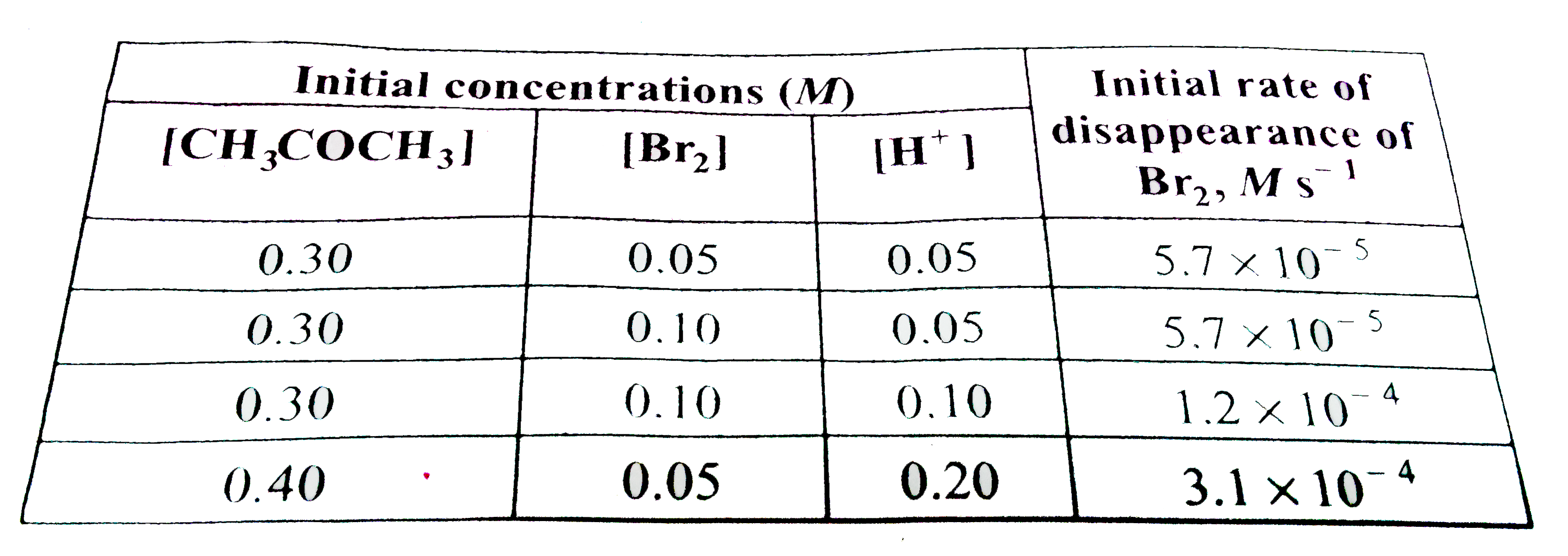
- The bromination of acetone that occurs in acid solution is represente...
Text Solution
|
- For the reaction, N2O5(g) rarr 2NO2(g)+1//2O2(g), the value of rate of...
Text Solution
|
- For a reaction, 2N(2)O(5)(g) to 4NO(2)(g) + O(2)(g) rate of reaction i...
Text Solution
|
- For reaction aArarrxP when [A] =2.2 m M, the rate was found to be 2.4 ...
Text Solution
|
- For the chemical reaction N(2)(g)+3H(2)(g)hArr2NH(3)(g) The correc...
Text Solution
|
- The rate of reaction. 2N(2)O(5) to 4NO(2) + O(2) can be written in...
Text Solution
|
- A subtance 'A' decomposes by a first-order reaction starting initially...
Text Solution
|
- The reaction A toB follows first order reaction. The time taken for 0....
Text Solution
|
- For a first order reaction, we obtain a straight line with positive sl...
Text Solution
|
- The rate constant k, for the reaction N(2)O(5)(g) rarr 2NO(2) (g) + (1...
Text Solution
|
- For the raction A+Brarr products, it is found that order of A is 2 and...
Text Solution
|
- The data for the reaction: A + B overset(k)rarr C. |{:("Experiment",...
Text Solution
|
- The reaction obey I order with respect to H(2) and ICl both. H(2) (g...
Text Solution
|
- Which of the following rate laws has an overall order of 0.5 for react...
Text Solution
|
- The alkaline hydrolysis of ethyl acetate is represented by the equatio...
Text Solution
|
- For the reaction A + B rarr products, doubling the concentration of A ...
Text Solution
|
- If the rate of the reaction is equal to the rate constant , the order ...
Text Solution
|
- The rate of reaction between two reactants A and B increases by a fact...
Text Solution
|