A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL KINETICS-PAST YEARS QUESTIONS
- If the rate of the reaction is equal to the rate constant , the order ...
Text Solution
|
- The rate of reaction between two reactants A and B increases by a fact...
Text Solution
|
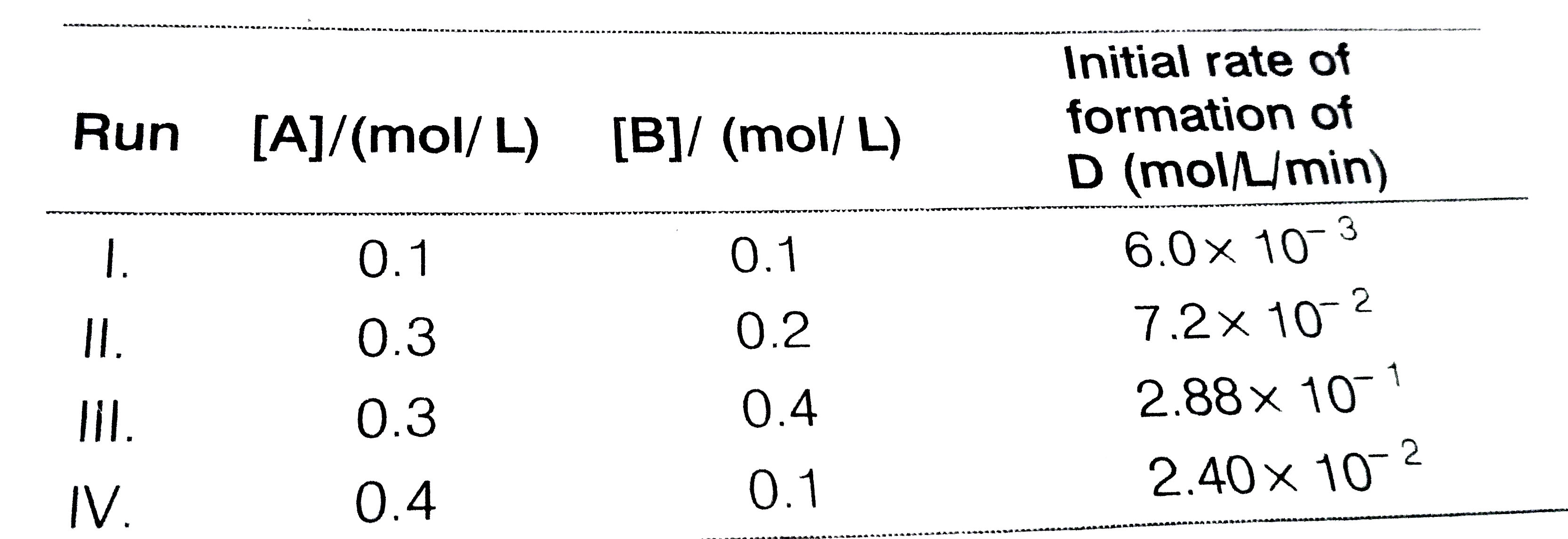
- During the kinetic study of the reaction, 2A+BtoC+D, following resul...
Text Solution
|
- The rate constant of the reaction A rarr B is 0.6 xx 10^(-3) mole per ...
Text Solution
|
- The decomposition of phosphine [PH(3)] on tungsten at low pressure is ...
Text Solution
|
- A first-order reaction which is 30% complete in 30 minutes has a half-...
Text Solution
|
- In a first order reaction A to B, if k is rate constant and initial co...
Text Solution
|
- The rate of a first order reaction is 1.5 xx 10^(-2) mol L^(-1) "min"^...
Text Solution
|
- For a first order reaction A rarrB , the reaction rate at reactant con...
Text Solution
|
- When initial concentration of a reactant is doubled in a reaction, its...
Text Solution
|
- The rate constant of a reaction is 0.69 xx 10^(-1)min^(-1) and the ini...
Text Solution
|
- A first order reaction is half completed in 45 minutes. How long does ...
Text Solution
|
- The half life of a substance in a certain enzyme catalyzed reaction is...
Text Solution
|
- T(50) of first order reaction is 10 min. starting with 10 "mol"^(-1) r...
Text Solution
|
- The half-life for the reaction N(2)O(5) to 2 NO(2) + (1)/(2) O(2) is 2...
Text Solution
|
- The rate of a first-order reaction is 0.04 mol L^(-1)s^(-1) at 10 sec...
Text Solution
|
- Mechanism of a hypothetical reaction X(2) + Y(2) rarr 2XY is given b...
Text Solution
|
- A first order reaction has a specific reaction rate of 10^(-2) sec^(-1...
Text Solution
|
- The correct difference between first and second order reactions is tha...
Text Solution
|
- When initial concentration of the reactant is doubled, the half-life p...
Text Solution
|