A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Cataylst and catalysis)|33 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Colloids, Emulsion, Gel and their properties with Application)|119 VideosSOLUTIONS
ERRORLESS|Exercise ASSERTION AND REASON|16 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SURFACE CHEMISTRY-ASSERTION AND REASON
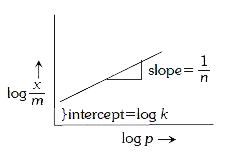
- For adsorption of a gas on a solid, the plot of log (x//m) vs log P is...
Text Solution
|
- Assertion : Colloidal sol scatters ight while true solution does not. ...
Text Solution
|
- Assertion : Colloidal particles show Brownian movement. Reason : B...
Text Solution
|
- Assertion : Soap acts as emulsifier in its cleansing action. Reason ...
Text Solution
|
- Assertion: Deep electric shock cause death of an animal Reason: Ele...
Text Solution
|
- Assertion: A catalyst is more effective in finely divided form. Reas...
Text Solution
|
- Assertion: NH(3) absorbs more readily over activted charcoal than CO(2...
Text Solution
|
- Assertion: Sky appears blue colour. Reason: Colloidal particles of d...
Text Solution
|
- Assertion: Physical absorption of molecular takes place on surface onl...
Text Solution
|
- Assertion : The micelle formed by sodium stereate in water has -COO^(-...
Text Solution
|
- Assertion: A quious gold colloidal solution is red in colour. Reason...
Text Solution
|
- Assertion :- An increase in surface area increases the rate of evapora...
Text Solution
|
- each question constain STATEMENT-1(Assertion ) and STATEMENT - 2 (reas...
Text Solution
|
- Assertion: Fe^(3+) can be used for coagulation of As(2)S(3) sol. Rea...
Text Solution
|
- each question constain STATEMENT-1(Assertion ) and STATEMENT - 2 (reas...
Text Solution
|