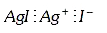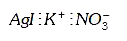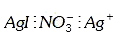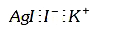A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
ERRORLESS|Exercise PAST YEARS QUESTIONS|27 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Cataylst and catalysis)|33 VideosSOLUTIONS
ERRORLESS|Exercise ASSERTION AND REASON|16 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SURFACE CHEMISTRY-NCERT BASED QUESTIONS (Colloids, Emulsion, Gel and their properties with Application)
- The dispersed phase in colloidal iron (III) hydroxide and collodial go...
Text Solution
|
- Which of the following process is not responsible for the presence of ...
Text Solution
|
- When a small quantity of aqueous solution of Kl is added to a very dil...
Text Solution
|
- Sedimentation potential is reverse of :
Text Solution
|
- When a freshly precipitated substance is converted into a colloidal so...
Text Solution
|
- Gold numbers of protective colloids A, B, C and D are 0.50, 0.01, 0.10...
Text Solution
|
- Purification of colloids is done by the process of
Text Solution
|
- The concentration of electrolyte required to coagulate a given amount ...
Text Solution
|
- Which of the following can protect a gold sol from coagulation by NaCl...
Text Solution
|
- Gold number is maximum for the lyophilic sol is
Text Solution
|
- Method by which lyophobic sol can be protected.
Text Solution
|
- Gold number is
Text Solution
|
- On addition of one mL solution of 10% NaCl to 10 mL gold sol in the pr...
Text Solution
|
- Gold number is associated with
Text Solution
|
- A negatively charged suspension of clay in water will need for precipi...
Text Solution
|
- Gelatian is mostly used in making ice cream in order to
Text Solution
|
- Lyophilic sols are more stable than lyophobic sols because
Text Solution
|
- The number of moles of lead nitrate needed to coagulate 2 mole of coll...
Text Solution
|
- Which of the following pairs of ions would be expected to form precipi...
Text Solution
|
- Statement : to stop bleeding from an injury ferric chloride can be app...
Text Solution
|