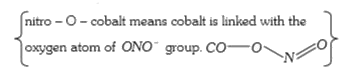A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (ISOMERISM)|1 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (WERNER.S COORDINATION THEORY)|16 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (NOMENCLATURE, OXIDATION STATE)
- Name the complex Ni(PF3)4
Text Solution
|
- The IUPAC name of [Co(NH3)6[Cr(C2O4)3] is
Text Solution
|
- As per IUPAC name , the name of the complex [Co(en)(2)(ONO)Cl]Cl is
Text Solution
|
- The oxidation state of cobalt in the following molecule is
Text Solution
|
- [Co(NH3)5Br]SO4 and [CO(NH3)5SO4]Br are examples of which type of isom...
Text Solution
|
- [CO(NH3)4Cl2]NO2 and [Co(NH3)4Cl*NO2]Cl are……………isomers
Text Solution
|
- Which of the following compounds exhibits linkage isomerism?
Text Solution
|
- The complexes [Co(NH3)6][Cr(C2O4)3] and [Cr(NH3)6][Co(C2O4)3] will h...
Text Solution
|
- [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2 are related to each other as
Text Solution
|
- Types of isomerism shown by [Cr(NH3)5NO2]Cl2 is
Text Solution
|
- [Fe(NO2)3Cl3] and [Fe(O-NO3Cl3] are
Text Solution
|
- [Cr(NH(3))(6)]Cr(SCN)(6) and [Cr(NH(3))(2)(SCN)(4)][Cr(NH(3))(4)(SCN)(...
Text Solution
|
- Which of the following can participate in linkage isomerism
Text Solution
|
- Octahedral complex
Text Solution
|
- Which of the following compounds shows optical isomerism?
Text Solution
|
- Indicate the complex ion which shows geometrical isomerism :
Text Solution
|
- Due to the presence of ambidenate ligands coordination compounds show ...
Text Solution
|
- [Co(NH3)5Br]SO4 and [Co(NH3)5SO4]Br are the examples of:
Text Solution
|
- What kind of isomerism exists between [Cr(H2O)6]Cl3, (violet) and [Cr(...
Text Solution
|
- Which one of the following will not show geometrical isomerism?
Text Solution
|