A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (ISOMERISM)|1 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (WERNER.S COORDINATION THEORY)|16 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (NOMENCLATURE, OXIDATION STATE)
- Which one of the following will not show geometrical isomerism?
Text Solution
|
- Draw isomers of the complex ion [Co(en)(2)Cl(2)]^(+)
Text Solution
|
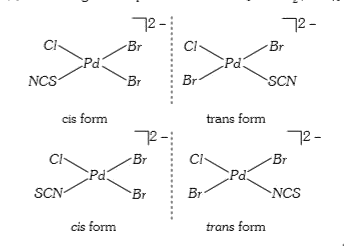
- The number of isomers possible for square planar complex K2[PdClBr2SCN...
Text Solution
|
- Number of possible isomers for the complex [Co(en)(2)CI(2)] will be (...
Text Solution
|
- In coordination compound [Co(en)(2)Cl(2)]Cl which is false
Text Solution
|
- Which of the following complex does not show geometrical isomerism
Text Solution
|
- Both geometrical and optical isomerism are exhibited by
Text Solution
|
- For the given complex [CoCl(2)(en)(NH(3))(2)]^(+), the number of geome...
Text Solution
|
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- The optically active co-ordination complex ion among the following is
Text Solution
|
- Which of the following has an optical isomer? (en=ethylenediamine) ?
Text Solution
|
- Which among the following statement are true for the complex [Co(NH(...
Text Solution
|
- The isomer is
Text Solution
|
- The number of geometical isomers possible for a square planar complex ...
Text Solution
|
- The total number possible isomers for the complex compound [Cu^(II)(NH...
Text Solution
|
- Which of the following does not have optical isomer
Text Solution
|
- Which of the following can exhibit geometrical isomerism
Text Solution
|
- Which one of the following complex is not expected to exhibit isomeris...
Text Solution
|
- Normally FeCl(3).6H(2)O consists of
Text Solution
|
- Which of the following complex does not show optical isomerism
Text Solution
|