A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (PREPARATION AND APPLICATION OF COORDINATION COMPOUNDS)|11 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (ORGANOMETALLIC COMPOUNDS)|13 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (CRYSTAL FIELD THEORY)|17 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-NCERT BASED QUESTION (COMPLEX STABILITY, SPECTROCHEMICAL SERIES AND EAN)
- The oxidation state and effective Atomic Number (EAN) of cobalt in [Co...
Text Solution
|
- If the Effective Atomic Number (EAN) of [A(NH3)6]Cl3 is 33, the atomic...
Text Solution
|
- The EAN of cobalt in Na(3)[Co(NO(2)(4)Cl(2)] is
Text Solution
|
- Effective atomic number of the central metal ion, Pt, in the complex ...
Text Solution
|
- Ag^(+) + NH3 iff [Ag(NH3)]^+ , k1 = 1.6 xx 10^3 [Ag(NH3)]^(+) + NH...
Text Solution
|
- Which of the following complexx ion has least stability
Text Solution
|
- Which of the following complexes formed by Cu^(2+) ions is most stable...
Text Solution
|
- The stabilization of coordination compound due to chelation is called ...
Text Solution
|
- CO is a stronger ligand than Cl^-, because
Text Solution
|
- Which one of the following is the correct order of field strength of l...
Text Solution
|
- The most stable coordination compound is
Text Solution
|
- Pick out the complex compound in which the central metal atom obeys EA...
Text Solution
|
- The metal ion in complex underline(A ) has EAN identical to t...
Text Solution
|
- The non -existant metal carbonyl among the following is
Text Solution
|
- Which of the following compounds is colourless
Text Solution
|
- The most stable complex among the following is
Text Solution
|
- In Spectrochemical series , chorine is above than water . I .e ...
Text Solution
|
- The most stable complex among the following is
Text Solution
|
- Which of the following spectrochemical series is TRUE ?
Text Solution
|
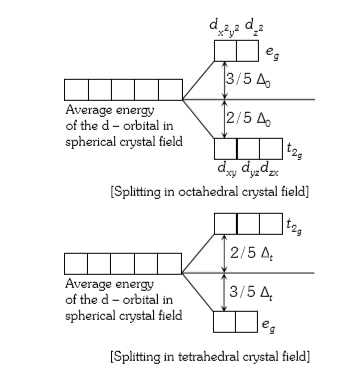
- The energies of d(xy) and d(z)^(2) orbits in octahedral and tetrahedr...
Text Solution
|