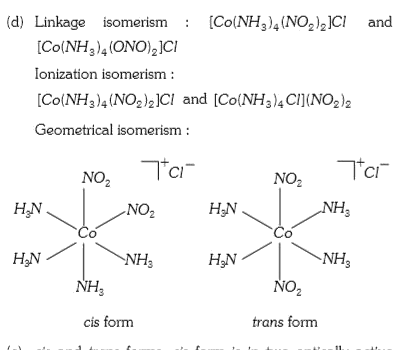
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (ORGANOMETALLIC COMPOUNDS)|13 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-PAST YEARS QUESTIONS
- Which of the following will show optical as well as geometrical isomer...
Text Solution
|
- Which of the following coordination compounds would exhibit optical is...
Text Solution
|
- [Co(NH3)4(NO2)92)]Cl exhibits .
Text Solution
|
- The number of possible isomers of an octahedral complex [Co(C2O4)2 (NH...
Text Solution
|
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- In which of the following pairs both the complex show optical isomeris...
Text Solution
|
- In a particular isomer of [Co(NH(3))(4)Cl(2)], the Cl-Co-Cl angle is 9...
Text Solution
|
- The type of isomerism shown by the complex [CoCl(2)(en)(2)] is
Text Solution
|
- Iron carbonyl, Fe(CO)5 is
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF(6)]^(3-) is
Text Solution
|
- The d-electron configurations of Cr^(2+), Mn^(2+), Fe^(2+) and Co^(2+)...
Text Solution
|
- Considering H2O as a weak field ligand, the number of unpaired electro...
Text Solution
|
- In [Ag(CN)2]^(-), the number of pi bonds is
Text Solution
|
- Which one of the following shows maximum paramagnetic character
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- Which of the following complex compounds will exhibit highest paramagn...
Text Solution
|
- Which among the following is a paramagnetic complex
Text Solution
|
- Which of the following is/are paramagnetic complex(es)?
Text Solution
|
- Which of these statements about [Co(CN)6]^(3-) is true?
Text Solution
|
- Which of the following is an inner orbital complex as well as diamagne...
Text Solution
|