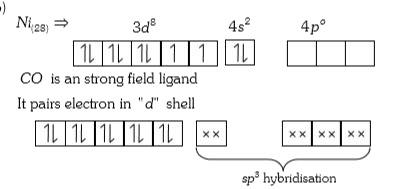
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise NCERT BASED QUESTION (ORGANOMETALLIC COMPOUNDS)|13 VideosCHEMISTRY IN EVERYDAY LIFE
ERRORLESS|Exercise Assertion & Reason|4 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-PAST YEARS QUESTIONS
- Pick out the correct statement with respect to [Mn(CN)(6)]^(3-):
Text Solution
|
- Which one of the following ions exhibits d-d transition and paramagnet...
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Text Solution
|
- Match the metal ions given in Column I with the spin magnetic moments ...
Text Solution
|
- Jahn - Teller effect is not observed in high spin complexes of
Text Solution
|
- The correct order for the wavelength of absorption in the visible regi...
Text Solution
|
- Correct increasing order for the wavelength of absorption in the visib...
Text Solution
|
- What is the correct electronic configuration of the central atom in K(...
Text Solution
|
- The correct increasing order of trans-effect of the following species ...
Text Solution
|
- CN^(-) is a strong field ligand. This is due to the fact that
Text Solution
|
- The ligands in anti- cancer drug cisplatin are
Text Solution
|
- An aqueous solution of COCL2 on addition of excess of concentrated HCl...
Text Solution
|
- The correct order of the stoichiometries of AgCl formed when AgNO3 in ...
Text Solution
|
- Which of the following is an organometallic compound?
Text Solution
|
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Which of the following carbonyls will have the strongest C-O bond
Text Solution
|
- Dimethyl glyoxime gives a red precipitate with Ni^(2+), which is used ...
Text Solution
|
- The formula of sodium nitroprusside is
Text Solution
|
- Which of the following has longest C-O bond length? (Free C-O bond len...
Text Solution
|
- An example of a sigma bonded organometallic compound is:
Text Solution
|