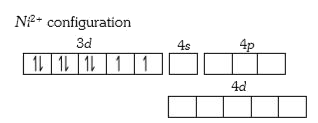
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-COORDINATION COMPOUNDS-ASSERTION AND REASON
- Assertion NF(3) is weaker ligands than N(CH(3))(3) Reason NF(3) ioni...
Text Solution
|
- Assertion: [Ni(en)(3)]Cl(2) has lower stability than [Ni(NH(3))(6)]Cl(...
Text Solution
|
- Assertion : H2N - NH2 is a chelating ligand. Reason : A chelating...
Text Solution
|
- These questions consist of two statements each, printed as Assertion a...
Text Solution
|
- Explain the following: (i) All the octahedral complexes of Ni^(2+)...
Text Solution
|
- Assertion : Potassium ferrocyanide is diamagnetic whereas potassium fe...
Text Solution
|
- Assertion: [Co(NO(2))(3) (NH(3))(3)] does not show optical isomerism. ...
Text Solution
|