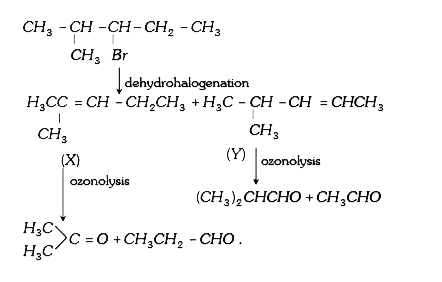
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HELOALKANES AND HALOARENES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dihalides, Trihalides, Tetrahalides, Unsaturated Halides )|46 VideosHELOALKANES AND HALOARENES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Haloarenes)|40 VideosHELOALKANES AND HALOARENES
ERRORLESS|Exercise Assertion & Reason|8 VideosGENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise ASSERTION & REASON|8 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ERRORLESS|Exercise Assertion and Reason|15 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-HELOALKANES AND HALOARENES-NCERT BASED QUESTIONS (Properties of Alkyl Halides)
- The hydrolysis of 2-bromo-3-methylbutane by S(N^(1)) mechanism gives m...
Text Solution
|
- Haloalkanes react with KCN to form alkyl cyanides as main product whil...
Text Solution
|
- 2-Bromobutane reacts wit OH^(-) in H(2)O to give 2-butanol. The reac...
Text Solution
|
- The odd decomposition of carbon chlorine bond form
Text Solution
|
- Which chlorine atom is more electronegative in the following ?
Text Solution
|
- Identify Z, in the following reaction. C(2)H(5)l overset("alc. KOH")...
Text Solution
|
- A primary alkyl halide would prefer to undergo :-
Text Solution
|
- Which of the following alkyl halides will undergo SN1 reaction most re...
Text Solution
|
- What should be the correct IUPAC name for diethylbromomethane?
Text Solution
|
- Chloromethane on treatment with excess of ammonia yields mainly
Text Solution
|
- Which of the following compounds will give racemic mixture on nucleoph...
Text Solution
|
- Ethyl bromide can be convertd into ethyl alcohol by
Text Solution
|
- Reaction of t-butyl bromide with sodium methoxide produces
Text Solution
|
- In presence of AICI(3) benzene and n-propyl bromide react in Friedel-C...
Text Solution
|
- CH(3)-CH(2)-Br on treatment with LiAlH(4) gives ethane gas while (CH(3...
Text Solution
|
- 2-bromopentane is heated with postassium ethoxide in ethano1 The major...
Text Solution
|
- In the solvolysis of 3-methyl-3-bromohexane, which of the following st...
Text Solution
|
- An alkyl halide with molecular formula C6H(13)Br on dehyrohalogenation...
Text Solution
|
- An organic compound A(C(4)H(6)CI) on reation withNa/diethyl ether giv...
Text Solution
|
- The major product of the following reaction is
Text Solution
|