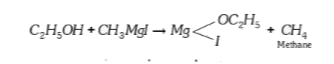A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dihydric, Trihydric and Unsaturated Alcohols)|21 VideosALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Nitrophenols, Dihydric and Trihydric Phenols)|15 VideosALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Uses of Alcohols, Phenols and Ethers)|14 VideosALDEHYDES AND KETONES
ERRORLESS|Exercise ASSERTION AND REASON|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ALCOHOLS, PHENOLS AND ETHERS-NCERT BASED QUESTIONS (Methanol and Ethanol)
- Which reagent can convert acetic acid into ethanol
Text Solution
|
- Conc. H(2)SO(4) reacts with C(2)H(5)OH at 170^(@)C to form
Text Solution
|
- Absolute alcohol can be prepared from rectified spirit by
Text Solution
|
- Ethyl alcohol exhibits acidic nature on reaction with
Text Solution
|
- When ethyl alcohol (C(2)H(5)OH) is mixed with ammonia and passed over ...
Text Solution
|
- A reaction between excess of C2H5OH and H2SO4 at 140^(@)C, gives
Text Solution
|
- The vapour pressure of aqueous solution of methanol is
Text Solution
|
- The process of manufacture of absolute alcohol from rectified spirit ...
Text Solution
|
- Methanol and ethanol are miscible in water due to
Text Solution
|
- The boiling point of methanol is greater than that of Methyl thiol bec...
Text Solution
|
- Ethyl alcohol cannot be used as solvent for methyl magnesium iodide be...
Text Solution
|
- Absolute alcohol can be obtained from rectified spirit
Text Solution
|
- Which of the following combination can be used to synthesise ethanol?
Text Solution
|
- A reaction between excess of C2H5OH and H2SO4 at 140^(@)C, gives
Text Solution
|
- Ethyl alcohol on oxidation with dil. acid and K2CrO7 gives
Text Solution
|
- Which is the most suitable method for removing the traces of water fro...
Text Solution
|
- The boiling point of ethanol should be less than that of
Text Solution
|
- If ethanol dissolves in water, then which of the following would be do...
Text Solution
|
- CH3CH2OH can be converted into CH3CHO by
Text Solution
|
- A sweet smelling compound formed by reacting acidic acid with ...
Text Solution
|