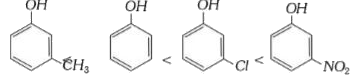
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Cyclic and Aromatic Ethers)|7 VideosALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Different Ethers and Hydroxy Compounds)|28 VideosALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dihydric, Trihydric and Unsaturated Alcohols)|21 VideosALDEHYDES AND KETONES
ERRORLESS|Exercise ASSERTION AND REASON|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ALCOHOLS, PHENOLS AND ETHERS-NCERT BASED QUESTIONS (Nitrophenols, Dihydric and Trihydric Phenols)
- Picric acid is
Text Solution
|
- Which of following is phenolic?
Text Solution
|
- Phenol, when it first reacts with concentrated sulphuric acid and then...
Text Solution
|
- The order of melting point of ortho, para, meta nitrophenol is
Text Solution
|
- Which of the following statement is not correct
Text Solution
|
- Which of the following compounds is most acidic ?
Text Solution
|
- The strongest acid among the following aromatic compounds is
Text Solution
|
- The increasing order of acidity among phenol, p-methylphenol, m-nitrop...
Text Solution
|
- Among the following , the compound that undergoes nitration readily is
Text Solution
|
- The correct order of acid strength of the following substituted phenol...
Text Solution
|
- Arrange the following compounds in the increasing order of their acidi...
Text Solution
|
- From the following figure, the correct observation is :-
Text Solution
|
- Mark the correct increasing order of reactivity of the following compo...
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Match the following Column A to Column B
Text Solution
|