A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-ALCOHOLS, PHENOLS AND ETHERS-PAST YEARS QUESTIONS
- At higher temperature, iodoform reaction is given by:
Text Solution
|
- What is the product obtained when chloride reacts with ethyl alcohol i...
Text Solution
|
- On heating glycerol with conc. H(2)SO(4) a compound is obtained which ...
Text Solution
|
- Glycerol reacts with P(4)+I(2) to form:
Text Solution
|
- When glycerol is treated with excess of Hl, the product formed is……………...
Text Solution
|
- o- nitrophenol is steam volatile while p-nitrophenol is not. Discuss.
Text Solution
|
- Which of the following will not be soluble in sodium hydrogen carbonat...
Text Solution
|
- The most suitable method of separation of a 1:1 mixture of o- and p- n...
Text Solution
|
- On boiling with concentrated hydrobromic acid, pheny1 Ethyl ether will...
Text Solution
|
- The ether that undergoes electrophilic substitution reactions is
Text Solution
|
- The reaction Can be classified as
Text Solution
|
- The compound which does not react with hydroxylamine is
Text Solution
|
- An organic compound X on treatment with pyridinium chlorochromate in d...
Text Solution
|
- Isopropylbenzene on air oxidation in the presence of dilute acid gives
Text Solution
|
- Which one of the following compounds has the most acidic nature ?
Text Solution
|
- Among the following sets of reactants which one produces anisole?
Text Solution
|
- Given are cyclohexanol (I), acetic acid (II),2,4,6-trinitrophenol (III...
Text Solution
|
- The heating of phenyl-methyl ethers with HI produces .
Text Solution
|
- The compound A on treatment with Na gives B, and with PCl(5) gives C. ...
Text Solution
|
- Anisole on cleavage with HI gives:
Text Solution
|
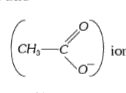 ion which is stabilized by only resonance. However, it is more resonance stabilized as compared to a phenoxide ion, thus more acidic as compared to phenol. 2, 4, 6 - trinitrophenol, however, is more acidic than acetic acid due to the presence of three electron withdrawing `-NO_2` groups.
ion which is stabilized by only resonance. However, it is more resonance stabilized as compared to a phenoxide ion, thus more acidic as compared to phenol. 2, 4, 6 - trinitrophenol, however, is more acidic than acetic acid due to the presence of three electron withdrawing `-NO_2` groups.