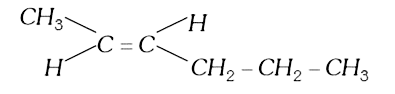
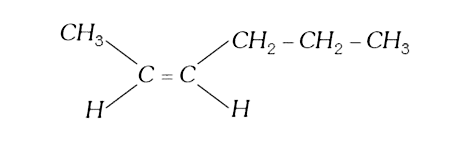
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
ERRORLESS|Exercise NCERT BASED QUESTION (AROMATIC HYDROCARBON)|78 VideosHYDROCARBONS
ERRORLESS|Exercise PAST YEAR QUESTIONS|88 VideosHYDROCARBONS
ERRORLESS|Exercise NCERT BASED QUESTION (ALKENE)|76 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosHYDROGEN
ERRORLESS|Exercise ASSERTION & REASON|6 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-HYDROCARBONS-NCERT BASED QUESTION (ALKYNE)
- What is the major product of the following reaction ? CH(3)C-=C-CH(2)...
Text Solution
|
- A compound C5H8 which gives white ppt. with ammonical AgNO3.A give (CH...
Text Solution
|
- Identify X in the following sequence of reactions CH(3) - underset(...
Text Solution
|
- Which one of the following characteristics apply to both ethene and et...
Text Solution
|
- A gas decolourises KMnO4 solution but gives no precipitate with ammoni...
Text Solution
|
- Acetylenic hydrocarbons are acidic because
Text Solution
|
- Lewisite, a chemical weapon, is prepared by the reaction of acetylene ...
Text Solution
|
- Acetylene can be prepared from
Text Solution
|
- A compound is treated with NaNH(2) to give sodium salt. Identify the c...
Text Solution
|
- KMnO4 will oxidised acetylene to
Text Solution
|
- Which of the following reagents distinguish ethylene from acetylene?
Text Solution
|
- If acetylene is passed through an electric arc in the atmosphere of ni...
Text Solution
|
- Acetylene is prepared industrially by passing electric discharge throu...
Text Solution
|
- When acetylene is passed into dilute sulphuric acid containing Hg^"2+...
Text Solution
|
- Acetylene adds on to HCN gives
Text Solution
|
- What happens when a mixture of acetylene and hydrogen is passed over h...
Text Solution
|
- Catalyst used in dimerisation of acetylene to prepare chloroprene is
Text Solution
|
- Which of the following will be the final product when C2H2 reacts with...
Text Solution
|
- When acetylene is treated with very dil. HCl in the presence of HgCl2,...
Text Solution
|
- Excess of CH(3)COOH is reacted with CH-=CH in presence of Hg^(2+), the...
Text Solution
|