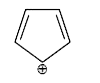
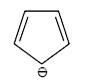
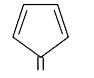
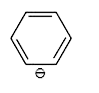
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
ERRORLESS|Exercise PAST YEAR QUESTIONS|88 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 VideosHYDROCARBONS
ERRORLESS|Exercise NCERT BASED QUESTION (ALKYNE)|67 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosHYDROGEN
ERRORLESS|Exercise ASSERTION & REASON|6 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-HYDROCARBONS-NCERT BASED QUESTION (AROMATIC HYDROCARBON)
- Decreasing order C-C bond length is (I) C(2)H(4) , (II) C(2)H(2) (II...
Text Solution
|
- Which of the following is aromatic ?
Text Solution
|
- Which of the following species is aromatic
Text Solution
|
- Which is a non-aromatic compound
Text Solution
|
- Which of the following is non-aromatic?
Text Solution
|
- What happens when napthalene balls are put inside kerosene
Text Solution
|
- In the following reaction, the product 'R' is: CaC(2) overset(H(2)O)...
Text Solution
|
- Which of the following possesses highest melting point ?
Text Solution
|
- Benzene reacts with CH3COCl in the presence of AlCl3 to give
Text Solution
|
- Nitration of benzene by nitric acid and sulphuric acid is
Text Solution
|
- The reaction of benzene with chlorine in the presence of iron gives
Text Solution
|
- Friedel-Crafts reaction using MeCI and anhydrous AICI(3) will take pla...
Text Solution
|
- On heating a mixture of sodium benzoate and sodalime, the following is...
Text Solution
|
- Benzene can be obtained in the reaction
Text Solution
|
- Benzene reacts with CH(3)Cl in the presence of anyhydrous AlCl(3) to f...
Text Solution
|
- Benzene is prepared in laboratory from which one of the following comp...
Text Solution
|
- Electrophile in the case of chlorination of benzene in presence of FeC...
Text Solution
|
- Benzne can be obtained by heating either benzoic acid with X or phenol...
Text Solution
|
- Find the major product in the following reaction
Text Solution
|
- Which among the following is very strong o-p-directing groups?
Text Solution
|