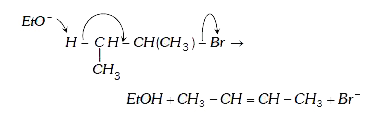A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-HYDROCARBONS-ASSERTION & REASON
- Assertion : Cyclopentadienyl anion is much more stable than allyl anio...
Text Solution
|
- Assertion : Tropylium cation is aromatic in nature Reason : The only...
Text Solution
|
- Assertion:Cyclobutane is less stable than cyclopentane. Reason : Pre...
Text Solution
|
- Assertion : Pyrrole is an aromatic heterocyclic compound Reason : ...
Text Solution
|
- Assertion : CH(4) does not react Cl(2) in dark. Reason: Chlorinat...
Text Solution
|
- Assertion : Alkyl benzene is not prepared by Friedel- Crafts alkylatio...
Text Solution
|
- Assertion : 2-Bromobutane on reaction with sodium ethoxide in ethanol ...
Text Solution
|
- Assertion: Styrence on reaction with HBr gives 1-bromo-1-phenylethane ...
Text Solution
|
- Assertion :Iodination of alkanes is reversible Reason:Iodination is ...
Text Solution
|
- Assertion:Freezing point of neopentane is more than n-pentane. Reaso...
Text Solution
|
- Assertion : The presence of Ag^(+) enhance the solubility of alkenes i...
Text Solution
|
- Assertion : Propene reacts with HBr in presence of benzoyl peroxide to...
Text Solution
|
- A) Addition of HBr on 2-butene gives two isomeric products. R) Addit...
Text Solution
|
- Assertion:Aryl halides are less reactive towards substitution of halog...
Text Solution
|
- Assertion:Benzene is a solvent for the Friedel Craft's alkylation of b...
Text Solution
|
- Assertion : [10]Annulene is not aromatic though it contains Huckel num...
Text Solution
|
- Assertion : Benzene forms benzene sulphonic acid with fuming H2SO4 at ...
Text Solution
|
- Assertion :Activating groups are electron donors. Reason: Nitroso gr...
Text Solution
|