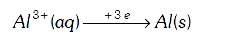A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (FARADAY.S LAW OF ELECTROLYSIS)|54 VideosELECTROCHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (CONDUCTOR AND CONDUCTANCES)|37 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosGENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise ASSERTION & REASON|8 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ELECTROCHEMISTRY-Assertion & Reason
- The quantity of charge required to obtain one mole of aluminium from A...
Text Solution
|
- Assertion : Zn metal is formed when a Cu plate in dipped in ZnSO(4) so...
Text Solution
|
- With increase in temperature the electrical conduction of metallic con...
Text Solution
|
- Assertion : A larger dry cell has higher emf. Reason : The emf of a ...
Text Solution
|
- Assertion(A): Whne acidified ZnSO(4) solution is electrolyzed between ...
Text Solution
|
- Assertion (A) : Galvanized iron does not rust. Reason (R): Zn has a...
Text Solution
|
- Assertion: E.m.f. and potential difference are same for cell. Reason...
Text Solution
|
- Assertion (A): For a Daniell cell : Zn|Zn^(2+)||Cu^(2+)|Cu with E(ce...
Text Solution
|
- Assertion: The cell potential of mercury cell is 1.35V which remains c...
Text Solution
|
- The questions consist of two atatements each, printed as Assertion and...
Text Solution
|
- Assertion: In the electrolysis of aqueous NaCl, Na is preferentially d...
Text Solution
|
- Consider the statements S1 and S2: S1: Conductivity always increases...
Text Solution
|