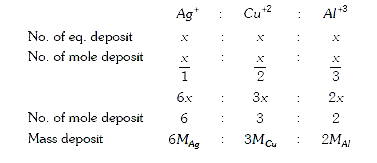A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (CONDUCTOR AND CONDUCTANCES)|37 VideosELECTROCHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (CELL CONSTANT AND ELECTROCHEMICAL CELLS) |38 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosGENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise ASSERTION & REASON|8 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ELECTROCHEMISTRY-NCERT BASED QUESTIONS (FARADAY.S LAW OF ELECTROLYSIS)
- Two electrolytic cells containing molten solutions of nickel chloride ...
Text Solution
|
- How many Faradays are needed for reduction of 2.5 mole of Cr(2)O(7)^(2...
Text Solution
|
- On passing C ampere of current for time t sec through 1 litre of 2(M) ...
Text Solution
|
- In the electrolysis of fused salt, the weight of the substance deposit...
Text Solution
|
- A current was passed for two hour through a solution of an acid that l...
Text Solution
|
- When an electric current is passed through acidified water, 112ml of H...
Text Solution
|
- The volume of H(2) gas at NTP obtained by passing 4 amperes through ac...
Text Solution
|
- An electrolytic cell contains a solution of Ag(2)SO(4) and have platin...
Text Solution
|
- Silver is removed electrolytically from 200mL of a 0.1N solution of Ag...
Text Solution
|
- How many coulombs are required for the oxidation of 1 mol of H(2)O to ...
Text Solution
|
- 9.65C of electric current is passed through fused anhydrous magnesium ...
Text Solution
|
- The quantitiy of electricity required to liberate 112 cm^(3) of hydrog...
Text Solution
|
- When 1 F of electricity is passed through acidulated water O(2) ev...
Text Solution
|
- In the electrolysis of water, one faraday of electrical energy would e...
Text Solution
|
- When a quantity of electricity is passed through CuSO(4) solution, 0.1...
Text Solution
|
- When a quantity of electricity is passed through CuSO(4) solution, 0.1...
Text Solution
|
- The charge required for the reduction of 1 mole of Cr(2)O(7)^(2-) ions...
Text Solution
|
- A current of 10.0A is passed through 1.0L of 1.0M HCl solution for 965...
Text Solution
|
- How many coulombs are required in order to reduce 12.3 g of nitrob...
Text Solution
|
- A constant current (0.5 amp) is passed for 1 hour through (i) aqueous ...
Text Solution
|