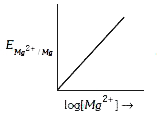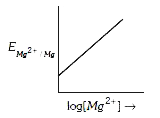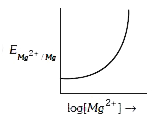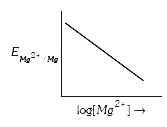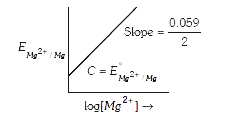A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- Electrode potential for Mg electrode varies according to the equation ...
Text Solution
|
- Electrode potential for Mg electrode varies according to the euation...
Text Solution
|
- Electrode potential of Mg- electrode varies as - E(Mg^(2+)|Mg)^(@)=E(M...
Text Solution
|
- Calculate E("cell")^(@) of the cell Mg|Mg^(2+)||Cu^(+)|Cu Given : E(Mg...
Text Solution
|
- Mg^(2+)//Mg इलेक्ट्रोड विभव क्या है जबकि उसे जो विलयन में डुबोया जाता ...
Text Solution
|
- Electrode potential equation for Mg electrode E(Mg^(2+)|Mg) = E(Mg^(2+...
Text Solution
|