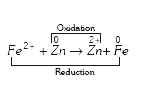A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 VideosELECTROCHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Corrosion)|8 VideosCOORDINATION COMPOUNDS
ERRORLESS|Exercise ASSERTION AND REASON|7 VideosGENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise ASSERTION & REASON|8 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ELECTROCHEMISTRY-PAST YEARS QUESTIONS
- An electrochemical cell is shown below Pt, H(2)(1 "atm")|HCl(0.1 M)|C...
Text Solution
|
- Consider the following relations for emf of a electrochemical cell (...
Text Solution
|
- The standard oxidation potentials, E^(@), for the half reactions are a...
Text Solution
|
- If E(Fe^(2+))^(@)//Fe = -0.441 V and E(Fe^(3+))^(@)//Fe^(2+) = 0.771...
Text Solution
|
- Standard free energies of formation (I kJ //mol ) at 298 K are -237 ....
Text Solution
|
- The standard redox potentials for the reactions, MN^(2+) + 2e^(-)to M...
Text Solution
|
- For the cell reaction Cu^(2+)(C(1),aq.)+Zn(s)hArrZn^(2+)(C(2),aq)+Cu...
Text Solution
|
- For the reduction of silver ions with copper metal, the standard cell ...
Text Solution
|
- The emf of a Daniell cell at 298 K is E(1) Zn|ZnSO(4)(0.01 M)||CuSO(...
Text Solution
|
- A hydrogen gas electrode is made by dipping platinum wire in a solutio...
Text Solution
|
- The pressure of H(2) required to make the potential of H(2^(-)) electr...
Text Solution
|
- The oxidation potential of a hydrogen electrode at pH = 10 and P(H(2))...
Text Solution
|
- Standard reduction potentails of the half reactions are given below: ...
Text Solution
|
- Four successive members of the first series of the transition metals a...
Text Solution
|
- On the basis of the following E^(@) values, the stongest oxidizing age...
Text Solution
|
- A solution contains Fe^(2+), Fe^(3+) and T^(-) ions. This solution was...
Text Solution
|
- The emf of a Daniell cell at 298 K is E(1) Zn|ZnSO(4)(0.01 M)||CuSO(...
Text Solution
|
- If the E(cell)^(@) for a given reaction has a positive value, then whi...
Text Solution
|
- For the cell reaction: 2Fe^(3+)(aq)+2l^(-)(aq)to2Fe^(2+)(aq)+l(2)(aq...
Text Solution
|
- For a cell involving one electron E(cell)^(0)=0.59V and 298K, the equi...
Text Solution
|