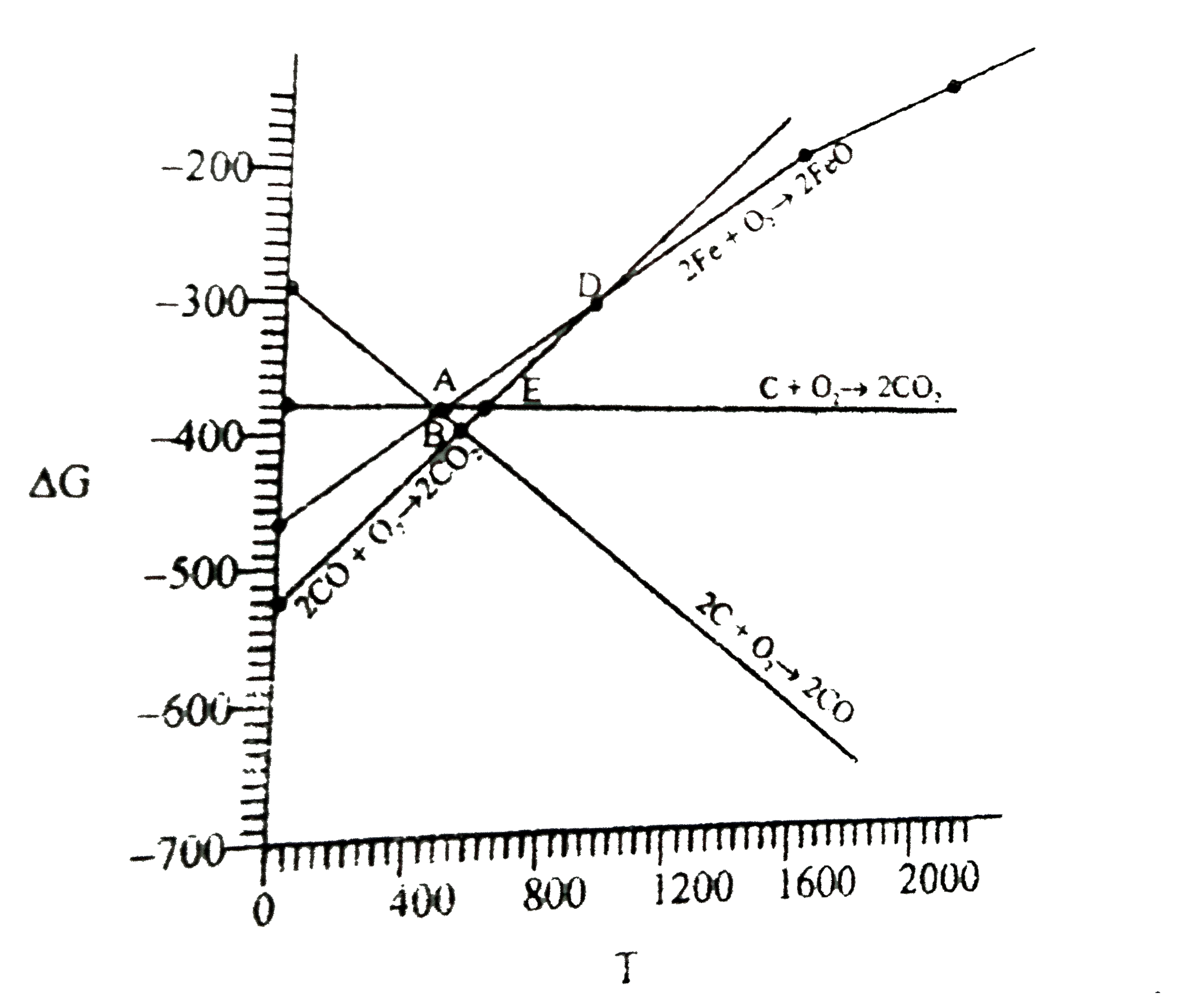
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Reduction of Free Metal)|43 VideosGENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Refining of Crude Metal)|27 VideosGENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS
ERRORLESS|Exercise NCERT BASED QUESTIONS (CONCENTRATION)|21 VideosELECTROCHEMISTRY
ERRORLESS|Exercise Assertion & Reason|11 VideosHELOALKANES AND HALOARENES
ERRORLESS|Exercise Assertion & Reason|8 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL PRINCIPLES & PROCESSES OF ISOLATION OF ELEMENTS-NCERT BASED QUESTIONS (ROASTING & CALCINATION)
- Roasting is done generally in case of
Text Solution
|
- Heating of pyrites in air for oxidation of sulphur is called Heating...
Text Solution
|
- The reaction 2ZnS +3O(2) rarr2ZnO +2SO(2) in the metallurgical process...
Text Solution
|
- Roasting is done in
Text Solution
|
- Roasting of copper pyrites is done:
Text Solution
|
- A metal obtained directly by roasting of its sulphide ore is
Text Solution
|
- Calcination is used in matallurgy for removal of
Text Solution
|
- Which of the following processes involves smelting ?
Text Solution
|
- A Substance which reacts with gangue to form fusible material is calle...
Text Solution
|
- Refractory metals are used in construction of furnances because
Text Solution
|
- The underlying of blast furnace is made of
Text Solution
|
- Which of the following substance can be used for drying neutral or bas...
Text Solution
|
- In the modern blast furnaces, the charge consists of a mixture of
Text Solution
|
- In a lime kiln, to get higher yield of CO(2) the measure that can be t...
Text Solution
|
- During the extraction of gold the following reactions takes place Au...
Text Solution
|
- The reduction of zinc oxide with coke occurs at temperature
Text Solution
|
- Choose the correct option of temperature at which carbon reduced FeO t...
Text Solution
|
- Find the area of figure given below.
Text Solution
|
- For the reduction of FeO at the temperature corresponding to point D, ...
Text Solution
|
- Direction (Q. Nos. 17-20) Answer the questions on the basis of figure ...
Text Solution
|