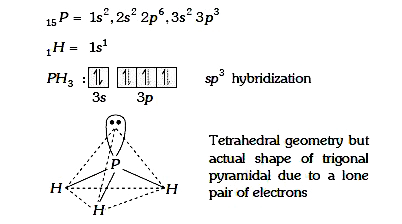
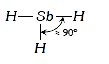A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Oxygen Family)|50 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Halogen Family)|73 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)-ASSERTION & REASON
- In Nitrogen family the H-M-H angle in the hydrides MH(3) gradually bec...
Text Solution
|
- Statement-1 : Liquid NH(3) is used for refrigeration. Statement-2 : ...
Text Solution
|
- Asseration:Although:Although PF(5),PCl(5) and PBr(5) are known, the pe...
Text Solution
|
- Assertion : PCl(5) is covalent in gaseous and liquid state but ionic i...
Text Solution
|
- Assertion : H(3)PO(3) is a diabasic acid. Reason: There are two H at...
Text Solution
|
- Statement-1 : Electrovalency of oxygen is two (O^(2-)) Statement-2 :...
Text Solution
|
- Assertion: Reaction of SO(2) and H(2)S in the presence of Fe(2)O(3) ca...
Text Solution
|
- Asseration: SeCl(4), does not havea tetrahedral structure. Reason: S...
Text Solution
|
- Assertion. Ozone is a powerful oxidising agent in comparison to O(2) ...
Text Solution
|
- Statement SO(2) can be used as reductant as well as oxidant. Explanat...
Text Solution
|
- Statement-1 : At room temperature oxygen exists as a diatomic gas, whe...
Text Solution
|
- Assertion. Nitrogen is less reactive than molecular oxygen. Reason. ...
Text Solution
|
- Assertion: N(2)H(4) cannot reduce S(2)O(3)^(2-) Reason : S(2)O(3)^(2...
Text Solution
|
- Assertion : Sb(2)S(3) is not soluble in yellow ammonium sulphide. Re...
Text Solution
|
- Assertion : The cyanide radical is a pseudo halide. Reason : The cy...
Text Solution
|
- Statement-1 : Chlorine and sulphur dioxide both are bleaching agents. ...
Text Solution
|
- Assertion : Halogens do not occur in free state. Reason : Halogens a...
Text Solution
|
- Assertion (A): The halogens absorb visible light. Reason (R ): All h...
Text Solution
|
- Asseration: F-F bond in F(2) molecule is strong. Reason: F-atom is s...
Text Solution
|
- Assertion : Chlorine has higher electron affinity than fluorine. Rea...
Text Solution
|
- Assertion : Xenon forms fluorides. Reason : Due to the strong elect...
Text Solution
|