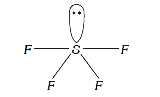A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Halogen Family)|73 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Noble Gases)|22 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)-NCERT BASED QUESTIONS (Oxygen Family)
- When conc. H2SO4 comes in contact with sugar it becomes black due to
Text Solution
|
- Which one is known as oil of vitriol ?
Text Solution
|
- Oleum is
Text Solution
|
- There is no S-S bond in
Text Solution
|
- Hypo is used in photography because of its
Text Solution
|
- Which of the following mixture is chromic acid?
Text Solution
|
- In the preparation of sulphuric acid, V(2)O(5) is used in the reaction...
Text Solution
|
- The molecular formula of sulphur is
Text Solution
|
- Conc. H(2)SO(4) is diluted
Text Solution
|
- SO(2)+H(2)S to Product. The final product is
Text Solution
|
- Sulphan' is
Text Solution
|
- Which of the following salts will not undergo hydrolysis in water?
Text Solution
|
- Hot conc. H(2)SO(4) acts as moderately strong oxidising agent. It oxid...
Text Solution
|
- The acid in which O-O bonding is present in :
Text Solution
|
- In the manufacture of sulphuric acid by contact process, Tyndall box i...
Text Solution
|
- In qualitative analysis when H(2)S is passed through an aqueous soluti...
Text Solution
|
- Which of the following is not tetrahedral in shape ?
Text Solution
|
- Which of the following are peroxoacids of sulphur ?
Text Solution
|
- The most efficient agent for the absorption of SO(3) is
Text Solution
|
- The oxidation number of sulphur is +4 in
Text Solution
|