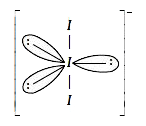
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Noble Gases)|22 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise PAST YEARS QUESTIONS|60 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Oxygen Family)|50 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)-NCERT BASED QUESTIONS (Halogen Family)
- Bromine is liberated when aqueous solution of potassium bromide is tre...
Text Solution
|
- In the manufacture of bromine from sea water the mother liquor contain...
Text Solution
|
- A one litre flask is full of brown bromine vapours. The intensity of b...
Text Solution
|
- Bromine water reacts with SO(2) to form
Text Solution
|
- The solubility of iodine in water increases in the presence of
Text Solution
|
- When thiosulphate ion is oxidised by iodine. which one of the followin...
Text Solution
|
- Colour of iodine solution is disappeared by shaking it with aqueous so...
Text Solution
|
- In K solution, I(2) readily dissolved and forms
Text Solution
|
- HI cannot be prepared by the action of conc. H(2)SO(4) on KI because
Text Solution
|
- When iodine is passed through aqueous solutions of NaF, NaBr and NaCl
Text Solution
|
- If I2 is dissolved in aqueous KI, the intense yellow species I3^ is fo...
Text Solution
|
- Conc. HNO3 reacts with I2 to form:
Text Solution
|
- Cl(2) reacts with CS(2) in presence of I(2) catalyst to form
Text Solution
|
- Ozone with dry iodine give
Text Solution
|
- Iodine is formed when potasium iodide reacts with:
Text Solution
|
- When chlorine gas is passed through an aqueous solution of KBr, the so...
Text Solution
|
- The pKa of oxoacids of chlorine in water follows the order
Text Solution
|
- Chlorine has two naturally occuring isotopes, ""^(35)Cl " and " ""^(3...
Text Solution
|
- The lattice energies of NaCl, NaF, KCl and RbCl follow the order
Text Solution
|
- Which of the following statements is not ture for halogens?
Text Solution
|