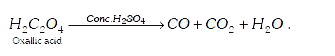A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Noble Gases)|22 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)-PAST YEARS QUESTIONS
- Shape of O(2)F(2) is similar to that of
Text Solution
|
- Nitrogen dioxide and sulphur dioxide have some properties in common, w...
Text Solution
|
- H2 SO4 acts as dehydrating agent in its reaction with
Text Solution
|
- Roasting of sulphides gives the gas X as a by product. This is a colou...
Text Solution
|
- Which of the following is not suitable for use in a desiccator to dry ...
Text Solution
|
- Peroxydisulphuric acid has which of the following bond ?
Text Solution
|
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- Sulphur trioxide can be obtained by which of the following reactions :
Text Solution
|
- In which pair of ions both the species contain S -S bond?
Text Solution
|
- Which is the correct thermal stability order of H(2)E(E=O,S,Se,Te and ...
Text Solution
|
- Which of the following statements is not true
Text Solution
|
- Metal halide which is insoluble in water is
Text Solution
|
- Which one of the following oxides is expected to exhibit paramagnetic ...
Text Solution
|
- Which one of the following orders is correct for the bond dissociation...
Text Solution
|
- Which of the statements given below is incorrect ?
Text Solution
|
- Which of the following has the lowest solubility
Text Solution
|
- The variation of the boiling points of the hydrogen halides is in the ...
Text Solution
|
- Which of the following is in the increasing order of the ionic charact...
Text Solution
|
- The mixture of concentrated HCl and HNO(3) made in 3:1 ratio contains
Text Solution
|
- On heating NaCl+K(2)CrO(7)+conc.H(2)SO(4), the gas comes out is
Text Solution
|