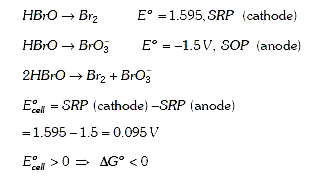A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise NCERT BASED QUESTIONS (Noble Gases)|22 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)-PAST YEARS QUESTIONS
- Which of the following has the lowest solubility
Text Solution
|
- The variation of the boiling points of the hydrogen halides is in the ...
Text Solution
|
- Which of the following is in the increasing order of the ionic charact...
Text Solution
|
- The mixture of concentrated HCl and HNO(3) made in 3:1 ratio contains
Text Solution
|
- On heating NaCl+K(2)CrO(7)+conc.H(2)SO(4), the gas comes out is
Text Solution
|
- Which of the following is used in the preparation of chlorine?
Text Solution
|
- Which one of the following is present as an active ingredient in bleac...
Text Solution
|
- Which two of the following are used for preparing iodised salt? (i) ...
Text Solution
|
- Tincture of iodine is :
Text Solution
|
- Which of the following pairs of compound is isoelectronic and isostruc...
Text Solution
|
- HgCl(2) and I(2) both when dissolved in water containing I^(-) ions th...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|
- The correct order of solubility in water for He,Ne,Ar,Kr,Xe, is
Text Solution
|
- When electric discharge is pressed through neon at low pressure, the c...
Text Solution
|
- Among the following molecules (i) XeO(3) (ii) XeOF(4) (iii) XeF(6) ...
Text Solution
|
- Match the Xenon compounds Column-I with its structure in Column -II an...
Text Solution
|
- Which of the following oxoacids of sulpher has -O-O- linkage ?
Text Solution
|
- Noble gases are named because of their inertness towards reactivity. I...
Text Solution
|
- Statement 1: Acid strength increases in the order given as HFltltHCllt...
Text Solution
|
- In which one of the following arrangements the given sequence is not s...
Text Solution
|
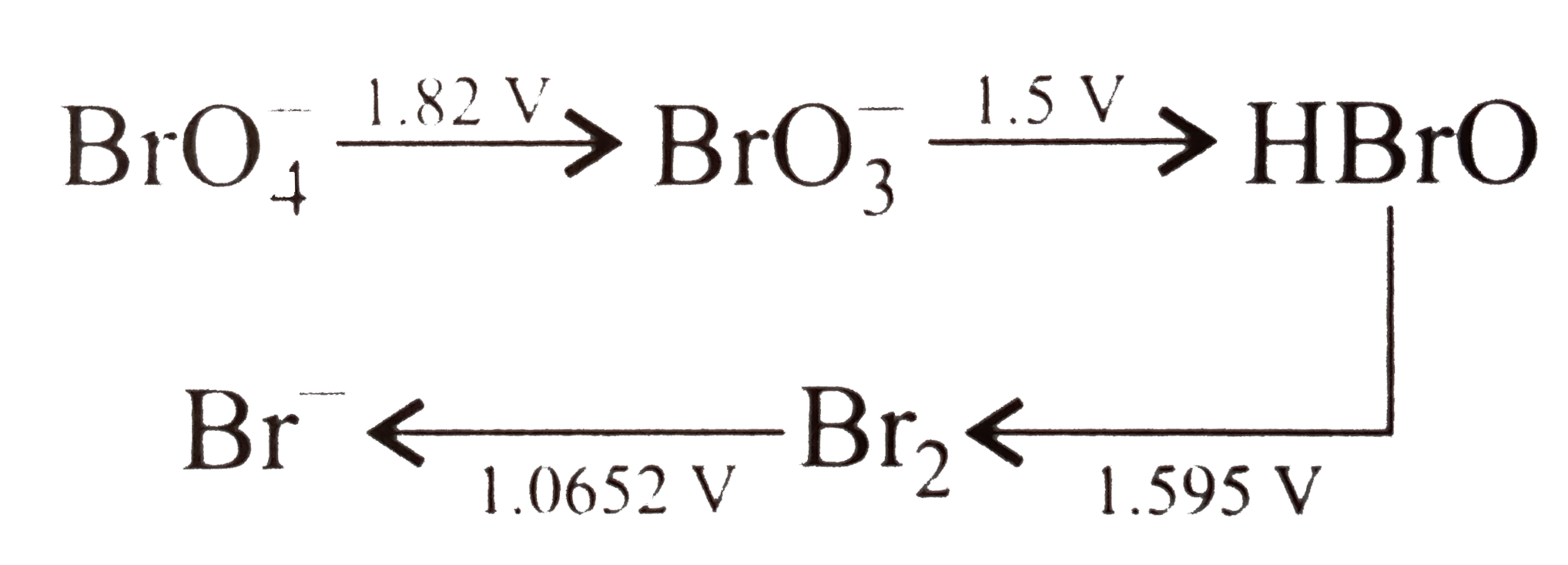 .
.