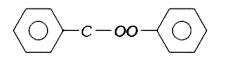
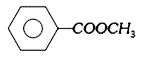
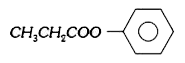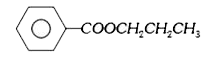A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
ERRORLESS|Exercise NCERT BASED QUESTIONS(FORMALDEHYDE)|6 VideosALDEHYDES AND KETONES
ERRORLESS|Exercise NCERT BASED QUESTIONS(ACETALDEHYDE)|14 VideosALDEHYDES AND KETONES
ERRORLESS|Exercise NCERT BASED QUESTIONS(PREPARATION OF ALDEHYDES AND KETONES)|36 VideosALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise Assertion & Reason|20 VideosBIOMOLECULES
ERRORLESS|Exercise ASSERTION & REASON |16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ALDEHYDES AND KETONES-NCERT BASED QUESTIONS(PROPERTIES OF ALDEHYDES AND KETONES)
- OHC-CHOoverset(OH^(-))toHOH(2)C-COOH. The reaction given is
Text Solution
|
- The correct order of reactivity of PhMgBr with underset((I))(Ph-unde...
Text Solution
|
- Claisen condensation is not given by
Text Solution
|
- Ketones reacting with Mg-Hg over water give
Text Solution
|
- A compound (A) C(5)H(10) Cl(2) on hydrolysis gives C(5)H(10)O which re...
Text Solution
|
- With which of the following reagents, carbonyl compound shows addition...
Text Solution
|
- The product of this reaction is
Text Solution
|
- Aldehydes that do not undergo aldol condensation are (1) propanal (...
Text Solution
|
- Anils are formed by the condensation of aniline with
Text Solution
|
- An organic compound 'A' burns with a sooty flame. It is negative towar...
Text Solution
|
- Which of the follwing compound does not react with concentrated alkali...
Text Solution
|
- An organic compound 'X' is oxidised by using acidified K(2)Cr(2)O(7). ...
Text Solution
|
- Which of the following will fail to react with potassium dichromate an...
Text Solution
|
- Aldehyde and ketones can decolourise
Text Solution
|
- CH-=CH underset("dil. "H2SO4)overset(HgSO4)to A underset(NaOH)overset(...
Text Solution
|
- The key step in cannizzaro's reaction is the intermolecular shift of
Text Solution
|
- In which of the following reactions, the product obtained is chiral ?
Text Solution
|
- One of the following named reaction is an example of "disproportionati...
Text Solution
|
- Which of the following reactions will not result in the formation of c...
Text Solution
|
- Ethylene is converted to X on passing through a mixture of acidified a...
Text Solution
|