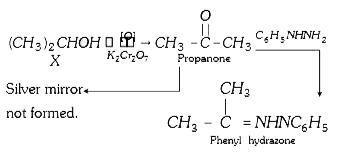A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
ERRORLESS|Exercise NCERT BASED QUESTIONS(BENZALDEHYDE)|17 VideosALDEHYDES AND KETONES
ERRORLESS|Exercise NCERT BASED QUESTIONS(ACETOPHENONE AND BENZOPHENONE)|10 VideosALDEHYDES AND KETONES
ERRORLESS|Exercise NCERT BASED QUESTIONS(ACETALDEHYDE)|14 VideosALCOHOLS, PHENOLS AND ETHERS
ERRORLESS|Exercise Assertion & Reason|20 VideosBIOMOLECULES
ERRORLESS|Exercise ASSERTION & REASON |16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ALDEHYDES AND KETONES-NCERT BASED QUESTIONS(ACETONE)
- Compound which gives acetone on ozonolysis
Text Solution
|
- Identify the reactant X and the product Y CH(3) - CO - CH(3) + X rar...
Text Solution
|
- The Grignard reagent, on reaction with acetone, forms :
Text Solution
|
- 3CH3 COCH3 overset(HCl)to (CH3)2 C =CH -CO -CH =C(CH3)2 This polymer...
Text Solution
|
- The reaction of acetone with PCl5 gives
Text Solution
|
- An important reaction of acetone is auto condensation in presence of c...
Text Solution
|
- What is the initial step in the reduction acetone by sodium borohydri...
Text Solution
|
- Pyrolysis of acetone gives CH(2)=C=O called :
Text Solution
|
- An organic compound 'X' is oxidised by using acidified K(2)Cr(2)O(7). ...
Text Solution
|
- When dihydroxy acetone reacts with HIO(4), the product is /are
Text Solution
|
- An organic compound 'A' has the molecular formula C(3)H(6)O, it underg...
Text Solution
|
- The enol form of acetone after treatment with D(2)O gives:
Text Solution
|