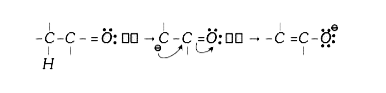A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-ALDEHYDES AND KETONES-ASSERTION AND REASON
- (A) Lower aldehydes and ketones are soluble in water but solubility de...
Text Solution
|
- Assertion: Acetaldehyde on treatment with alkali gives aldol. Reason...
Text Solution
|
- Assertion: Acetylene on treatment with alkaline KMnO(4) produce acetal...
Text Solution
|
- STATEMENT -I : Acetophenone and benzophenone can be distinguished by ...
Text Solution
|
- Assertion: Isobutanal does not give iodoform test. Reason : It does ...
Text Solution
|
- Assertion: Benzaldehyde is more reactive than ethanal towards nucleoph...
Text Solution
|
- Assertion : Aldol condensation can be catalysed both by acids and base...
Text Solution
|
- Assertion: Formaldehyde cannot be prepared by Rosenmund's reduction. ...
Text Solution
|
- Assertion : alpha-hydrogen atoms in aldehydes or ketones are acidic. ...
Text Solution
|
- Assertion: Hydroxyketones are not directly used in Grignard reaction. ...
Text Solution
|