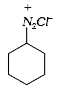
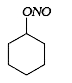
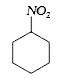
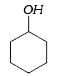
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
ERRORLESS|Exercise NCERT Based Questions (Different Nitrogen Containing Compounds)|26 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ERRORLESS|Exercise Past Years Questions|43 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
ERRORLESS|Exercise NCERT Based Questions (Aniline)|41 VideosHELOALKANES AND HALOARENES
ERRORLESS|Exercise Assertion & Reason|8 VideosPOLYMERS
ERRORLESS|Exercise ASSERTION & REASON|4 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ORGANIC COMPOUNDS CONTAINING NITROGEN-NCERT Based Questions (Diazonium Salts)
- The diazonium salts are the reaction products in presence of excess of...
Text Solution
|
- Azo dye is prepared by the coupling of phenol and:
Text Solution
|
- Dye test can be used to distinguish between
Text Solution
|
- The replacement of diazonium group by fluorine is known as
Text Solution
|
- The reaction Aroverset(+)N(2)Cl^(-) overset(Cu//HCl)to ArCl +N(2) + ...
Text Solution
|
- Production and productivity can be represented by ul(x) and ul(y) resp...
Text Solution
|
- Which will not go for diazotisation
Text Solution
|
- Identify the product in the following reaction. 3,4,5-tribromoanili...
Text Solution
|
- Action of NaNO(2) + dil HCl on ArNH(2) yields ArN(2)^(+)Cl^(-) A simil...
Text Solution
|
- Which of the following will be most stable diazonium salt RN2^(+)X^(-)...
Text Solution
|
- Which of the following compounds will not undergo azo coupling reactio...
Text Solution
|
- Diazo coupling is useful to prepare some :
Text Solution
|
- In the diazotisation of anline with sodium nitrite and hydrochloride a...
Text Solution
|
- The reagent (s) used for the conversion of benzene diazonium hydrogen ...
Text Solution
|