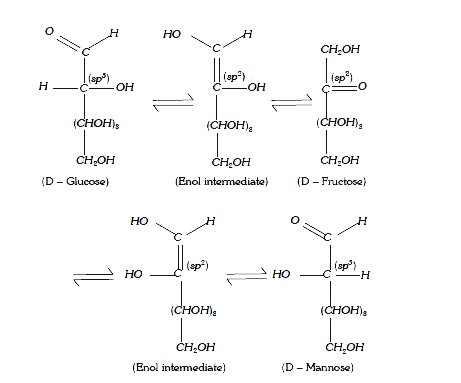
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-BIOMOLECULES -ASSERTION & REASON
- Assertion : Glycine is amphoteric in nature . Reason: Glycine contai...
Text Solution
|
- Assertion : sucrose is known as invert sugar Reason : sucrose is a di...
Text Solution
|
- Assertion: Proteins on hydrolysis produce amino acids. Reason : Amin...
Text Solution
|
- Assertion : Sucrose undergo mutarotation. Reason : Sucrose is a disa...
Text Solution
|
- Assertion : DNA molecules and RNA molecules are found in the nucleus o...
Text Solution
|
- Assertion: All amino acids exists as Zwitter ions. Reason: Amino aci...
Text Solution
|
- Assertion(A): Activity of an enzyme is pH dependent. Reason(R): Chan...
Text Solution
|
- Assertion : Glycosides are hydrolysed in acidic conditions. Reason ...
Text Solution
|
- Assertion : Haemoglobin is an oxygen carrier. Reason : Oxygen binds...
Text Solution
|
- Assertion. Carboxypetidase is an exopeptidase. Reason. It cleaves th...
Text Solution
|
- Assertion : Sucrose is a non- reducing sugar. Reason : It has glycos...
Text Solution
|
- Assertion : Sequence of bases in DNA is TGAACCCTT and sequence of base...
Text Solution
|
- Assertion: Solubilities of proteins is minimum at the isoelectronic po...
Text Solution
|
- Assertion : Amino acids are soluble in benzene and ether. Reason : A...
Text Solution
|
- Assertion : Treatment of D-glucose with alkali affords an equilibrium ...
Text Solution
|
- Assertion: Disruption of the natural structural of a protein is called...
Text Solution
|