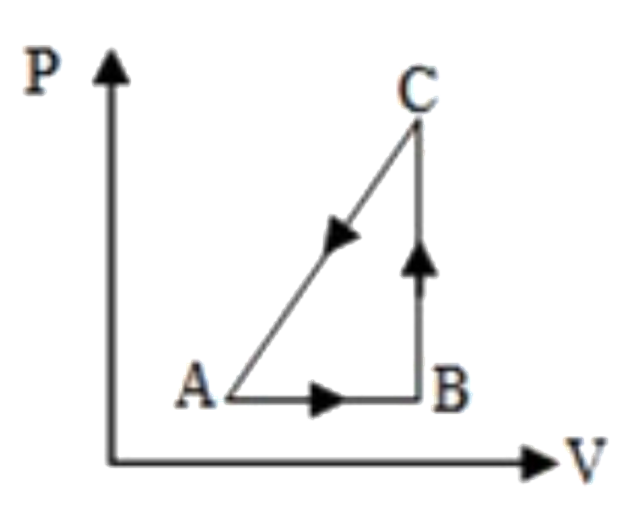
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The P - V diagram of a system undergoing thermodynamic transformation ...
Text Solution
|
- The P-V diagram of a system undergoing thermodynnaic transformation is...
Text Solution
|
- The P - V diagram of a system undergoing thermodynamic transformation ...
Text Solution
|
- For a gaseous system find change in internal energy if the heat suppli...
Text Solution
|
- If the amount of heat given to a system is 50 J and work done on the s...
Text Solution
|
- The P - V diagram of a system undergoing thermodynamic changes is as s...
Text Solution
|
- A system is provided with 50 J of heat and the work done on the system...
Text Solution
|
- The P - V diagram of a system undergoing thermodynamic transformation ...
Text Solution
|
- What is the change in internal energy if 10 J of heat is given to syst...
Text Solution
|