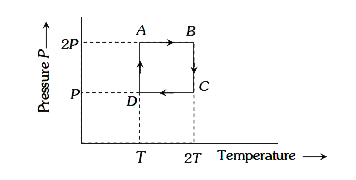
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of an ideal gas having initial volume V. pressure 2P and temp...
Text Solution
|
- Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as s...
Text Solution
|
- One mole of an ideal gas is taken through the cyclic process ABCDA, as...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic change ABCD. From the give...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in givend p-V dia...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA a...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic process ABCDA as shown in ...
Text Solution
|
- One mole of a monatomic ideal gas undergoes a cyclic process as shown ...
Text Solution
|