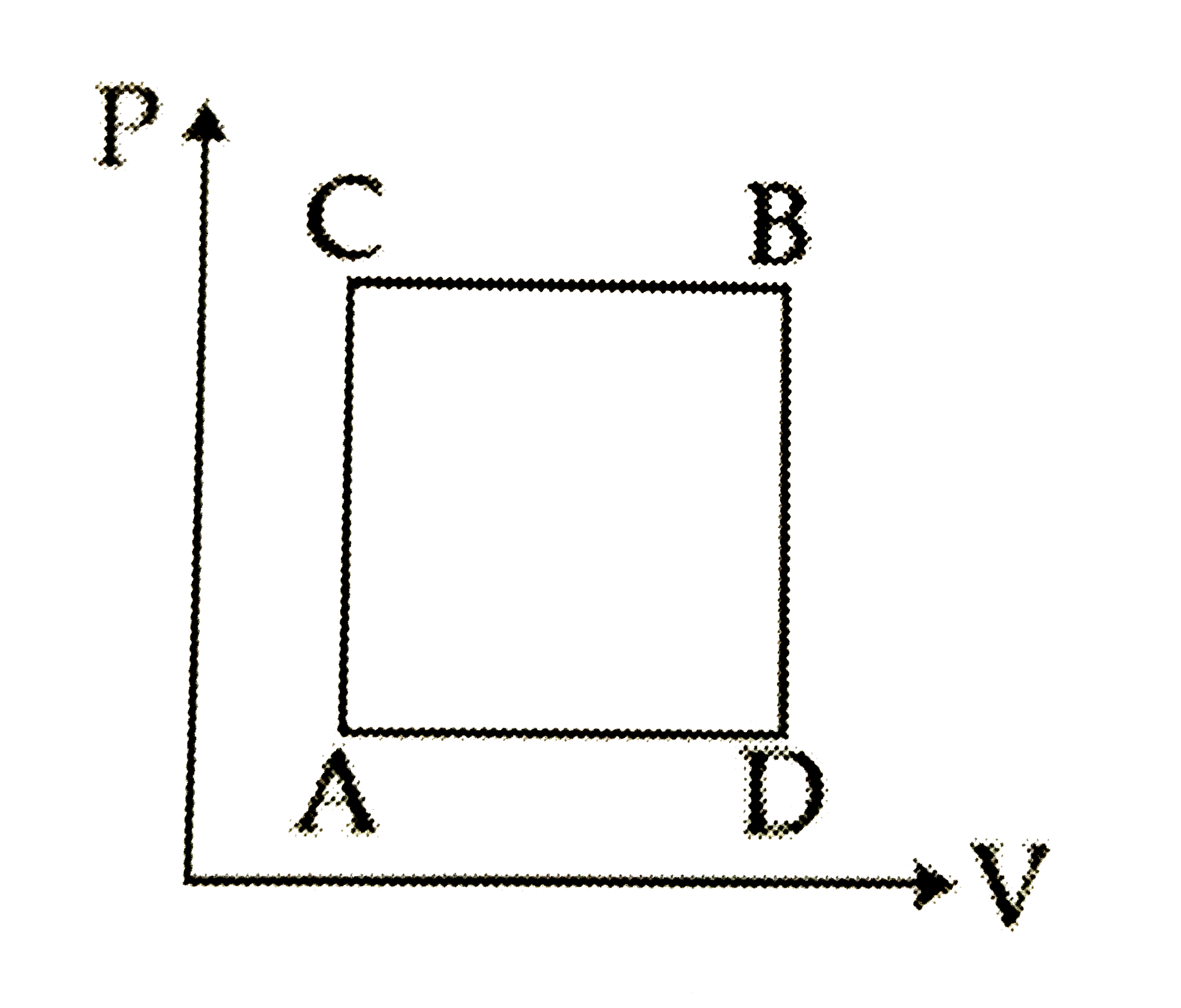
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A gas can be ken from A to B via two different processes ACB and ADB. ...
Text Solution
|
- In given figure, when a thermodynamic system is taken from state A to ...
Text Solution
|
- How much heat flows into the system along path ADB it the work done by...
Text Solution
|
- When a system is taken from state 'a' to state 'b' along the path 'acb...
Text Solution
|
- When a system is taken from state A and B along the path ACB, 80J of h...
Text Solution
|
- In given figure, when a thermodynamic system is taken from state A to ...
Text Solution
|
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- A gas can be ken from A to B via two different processes ACB and ADB. ...
Text Solution
|
- A gas can be taken from A to B via two different processes ACB and ADB...
Text Solution
|