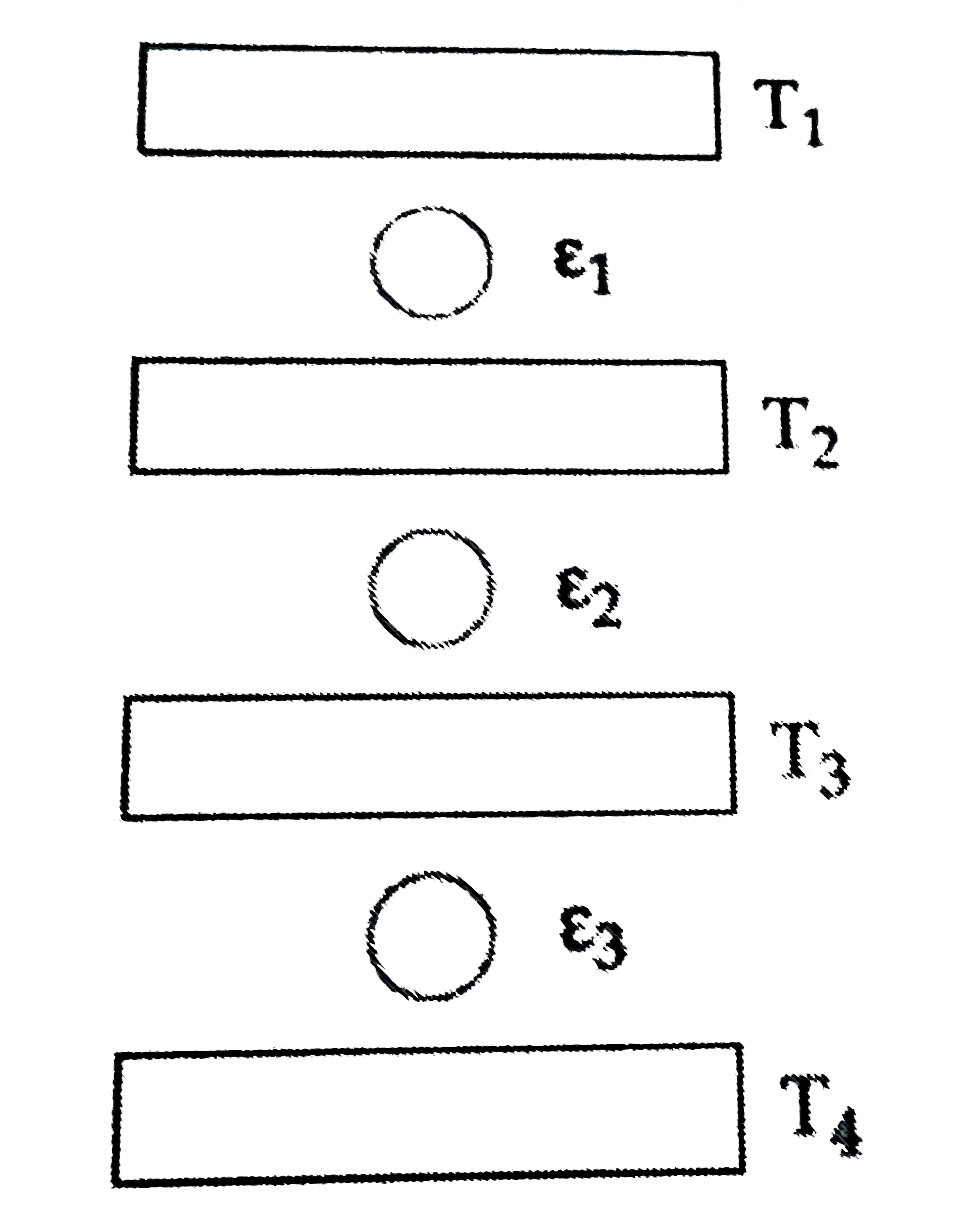
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Three Carnot engines operate in series between a heat source at a temp...
Text Solution
|
- An ideal heat engine working between temperature T(1) and T(2) has an ...
Text Solution
|
- What percentage T(1) is of T(2) for a 10% efficiency of a heat engine?...
Text Solution
|
- A heat engine works between a source and a sink maintaned at constant ...
Text Solution
|
- The efficiency of carnot's heat engine is 0.5 when the temperature of ...
Text Solution
|
- Three Carnot engines operate in series between a heat source at a temp...
Text Solution
|
- Two Carnot engines A and B are operated in series. The first one, A, r...
Text Solution
|
- Two ideal Carnot engines operate in cascade (all heat given up by one ...
Text Solution
|
- तीन कार्नो इंजन श्रेणीक्रम में T(1) तापमान के एक गर्म ऊष्मा भण्डार तथा...
Text Solution
|