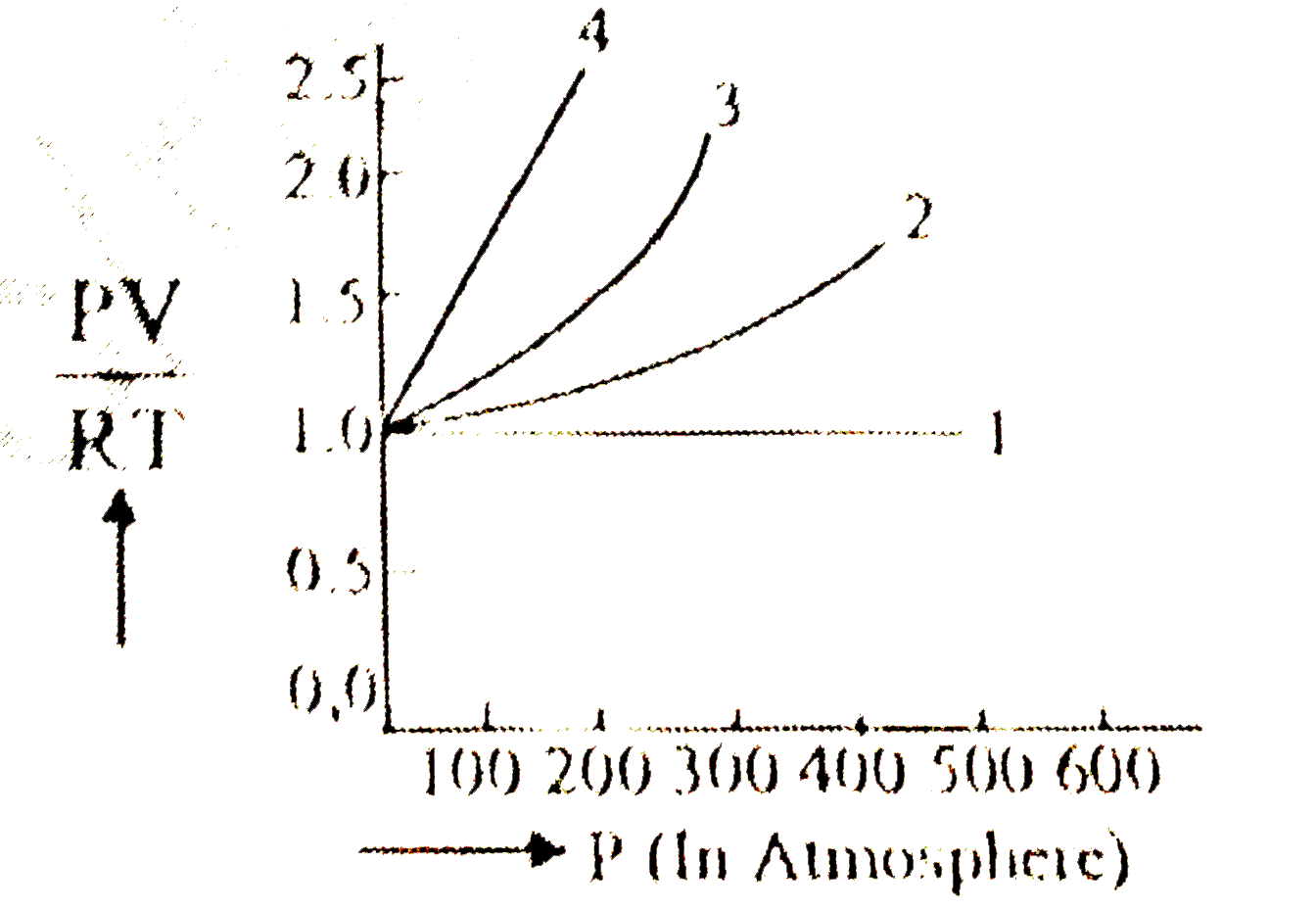A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A fixed amount of ideal gas (1 mole) is taken and is subjected to pres...
Text Solution
|
- The given curve represents the variation of temperature as a function ...
Text Solution
|
- Assertion : For a certain fixed amount of gas the product PV is always...
Text Solution
|
- A fixed amount of ideal gas (1 mole) is taken and is subjected to pres...
Text Solution
|
- Sketch shows the plot of Z v/s P for a hypothetical gas for one mole a...
Text Solution
|
- Statement-1 : At low pressure and high temperature real gas approaches...
Text Solution
|
- 1 मोल नाइट्रोजन गैस का उच्च ताप पर दाब P परिवर्तित करते हुए (PV)/(RT) ...
Text Solution
|
- वास्तविक गैसें, आदर्श गैस समीकरण PV=RT का केवल निम्न दाब व उच्च ताप पर...
Text Solution
|
- Which is correct in case of extent of physisorption High temperature l...
Text Solution
|